
����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⣮
(1)��֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��

��A�Ļ��ϼ�__B�Ļ��ϼ�(��������������������=��)��
(2)ʵ��֤����KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ����(��ͼ��ʾ)������3�����Ӿ���ľ������������±���

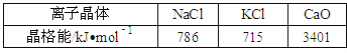
��� 4�����Ӿ���(������NaCl)�۵�Ӹߵ��͵�˳���ǣ�__��
(3)���������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�ã�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���___________________________.
(4)ij�����ķ��ӽṹ��ͼ��ʾ����Nԭ�ӵ��ӻ���ʽΪ__����̬Niԭ�ӵĵ����Ų�ʽ__��
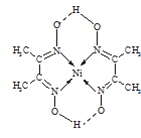
���𰸡��� TiN��MgO��CaO��KCl CrO2 sp2 [Ar]3d84s2
��������
��1��A��BΪ��������Ԫ�أ��ɵ��������ݿ�֪A��ʧȥ3�����ӣ�B��ʧȥ2�����ӣ�
��2�����Ӿ���ľ�����Խ���۵�Խ�ߣ�
��3���������ӵ������������жϣ�
��4�����ݷ��ӽṹ��֪N�γ�3��������1���м��������������ԭ����д�����Ų�ʽ��
��1��A��BΪ��������Ԫ�أ��ɵ��������ݿ�֪A��ʧȥ3�����ӣ�����ϼ�Ϊ+3�ۣ�B��ʧȥ2�����ӣ�����ϼ�Ϊ+2�ۣ�������ϼ�A>B��
�ʴ�Ϊ��>��
��2�����Ӿ�������Ӱ뾶ԽС���������Խ�࣬������Խ��������۷е�Խ�ߣ�����TiN>MgO��MgO>CaO���ɱ������ݿ�֪CaO>KCl����TiN>MgO>CaO>KCl��
�ʴ�Ϊ��TiN>MgO>CaO>KCl��
��3��V2O5�У�V����������ȫ��ʧȥ��ɼ�����δ�ɶԵ�������0��CrO2��Crʧȥ4�����������ӵ������δ�ɶԵ�����Ϊ2�����������ӵ�δ�ɶԵ���Խ�����Խ�������ʺ���¼�����ŷ�ԭ�ϵ���CrO2��
�ʴ�Ϊ��CrO2��
��4�����ݷ��ӽṹ��֪N�γ�3���ļ���1���м������ӻ�����Ϊsp2�ӻ���Ni��ԭ������Ϊ28��������Ų�ʽΪ[Ar]3d84s2��
�ʴ�Ϊ��sp2��[Ar]3d84s2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���˽Ⲣ�������ʵ��ڲ��ṹ�Լ���ѧ���;������Ϳ��������Ǹ��õ��˽��������硣��֪��ʵ������![]() ���Ȼ��⣬����ˮ�γ����ᡣ
���Ȼ��⣬����ˮ�γ����ᡣ
��1��д��ԭ�ӵĽṹʾ��ͼNa_______��Cl______��
��2��NaCl����ʽΪ___��������ѧ������_____������______���壻HCl����ʽΪ____��������ѧ������____������______���塣
��3���Ȼ�������ˮ�γ�����ĵ��뷽��ʽ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ��
��1����Ӧ��ѧ����ʽΪ��___��
��2����ⱥ��ʳ��ˮʱ��___����������ҺpH���
��3��ȡ��������Һ��ϵ��ʵ�飬���н����д������___��������ĸ��
A.�μ�ʯ���Լ�����Һ�ʺ�ɫ
B.�μ��Ȼ�þ��Һ���а�ɫ��������
C.����ʯ��ʯ�������ݲ���
D.�ȼ��������ᣬ�ٵμ���������Һ������������˵����Һ�к���������
��4����ⱥ��ʳ��ˮһ��ʱ�����Ҫ����Һ�ָ����ǰ��״̬��Ӧ����Һ�м��루��ͨ�룩һ������___������ĸ����
A.NaCl���� B.NaCl��Һ C.HCl���� D.����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������дӺ�ˮ�и�����������������ǴӺ�������ȡ�ⵥ�ʵ����̣�
����![]() ������
������![]() ����Һ
����Һ![]() ��I-����Һ
��I-����Һ![]() ��I2����Һ
��I2����Һ![]() ��I2�����Ȼ�̼��Һ
��I2�����Ȼ�̼��Һ![]() �����
�����
��1�������������У����ڹ��˲�������___����a��b��c��d��ʾ����ͬ����������ȡ��������___��
��2�������ձ����������ͱ���ļг���������ɹ��˲�����ȱ�ٵIJ���������___�������ȡ��������Ҫ�IJ���������___��
��3��д����I-����Һ��ͨ����������Ӧ�����ӷ���ʽ��___��
��4����I2��ˮ��Һ�м������Ȼ�̼�����ã��۲쵽��Һ�ֲ㣬�²���Һ��___��
��5�����Ȼ�̼�ܴӵ�ˮ����ȡ�����ԭ���Ǣ�___����___����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����е����Ų�ͼ��ÿһ��С�����ʾһ��ԭ�ӹ��������ʾԪ�ص�ԭ���У��������������״̬������ ��
A. ![]() B.
B. ![]()
C. ![]() D.
D. ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(NOC1)��һ�ֻ�ɫ���壬�е�Ϊ-5.5����Һ��״̬�ʺ��ɫ����ˮ��ˮ�⣬�������Ȱ������Ȼ������ɡ�ij��ѧ��ȤС�鰴��ͼװ����Cl2��NO�Ʊ�NOC1���ش�����������⣺

(1)����a��������_____,װ�ü��з�Ӧ�����ӷ���ʽ��______.
(2)װ���ҵ�������____,ʵ�鿪ʼʱ���ȴ���k,���۲쵽װ�ñ��г���____(������)ʱ�ٻ���ͨ��Cl2.
(3)װ�ñ�������NOC1�ķ�Ӧ����ʽ��___,װ�ö��б���ˮ��������______.
(4)����������װ���������һ������ȱ�ݣ��Ľ�������_____(����������)��
(5)��ҵ�Ƶõ�NOC1�г���������N2O4���ʣ�Ϊ�ⶨ��Ʒ���Ƚ�������ʵ�飺��ȡ1.441g��Ʒ����������NaOH��Һ�У����뼸��K2CrO4��Һ��ָʾ������0.8000mol/L�����ữ��AgNO3��Һ�ζ���������AgNO3��Һ�����Ϊ25.00mL,��NOC1����������Ϊ___%(����2λС��)�����AgNO3��Һ���ֲ��ֱ���(���ʵIJ��ֲ����뷴Ӧ)�����õ�NOC1������������____(����ƫ��������ƫС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. ��0.1 mol��L1�İ�ˮ�м���������粒��壬����Һ��![]() ����
����
B. �����£�0.1 mol��L1һԪ��(HA)��ҺpH=3�������Һ�У�c2(H+)=c(H+)��c(A)+KW
C. ����1 mol KAl(SO4)2����Һ�м���Ba(OH)2��Һ�õ����������ʵ������Ϊ 2 mol
D. ��Ca(ClO)2��Na2SO3��FeCl3��NaAlO2��Һ���ɾ��ò���ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���߷��Ӻ���J�ĺϳ�·�����£�

��֪��i��
ii��
��1��д��E�����ƣ�_____________����G����H�ķ�Ӧ����Ϊ��______________��
��2��I�й��������ƣ�____________________��B��C�ķ�Ӧ����Ϊ��___________��
��3����I�ɺϳ��л���K��K�к���3����Ԫ����д��I�ϳ�K�Ļ�ѧ����ʽ��____________________________________________________��
��4��D��E��Ӧ�Ļ�ѧ����ʽ��_____________________________��
��5���л���L��C9H10O3����������������ͬ���칹����______�֡�
����FeCl3��Һ������ɫ��Ӧ
����I������ͬ�Ĺ�����
�۱�������3��ȡ����
��6��������������е���Ϣ�������![]() �Ʊ�
�Ʊ� �ĺϳ�·��_____���ϳ�·������ͼʾ����
�ĺϳ�·��_____���ϳ�·������ͼʾ����![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�Ϊ2 L���ܱ������м�����������̿��NO��������Ӧ��C(s)+2NO(g)![]() N2(g)+CO2(g)����H<0��NO��N2�����ʵ����仯���±���ʾ��
N2(g)+CO2(g)����H<0��NO��N2�����ʵ����仯���±���ʾ��
���ʵ���/mol | T1/�� | T2/�� | |||||
0 | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | |
NO | 2.0 | 1.16 | 0.80 | 0.80 | 0.50 | 0.40 | 0.40 |
N2 | 0 | 0.42 | 0.60 | 0.60 | 0.75 | 0.80 | 0.80 |
��ش��������⣺
(1) 0��5 min�ڣ���CO2��ʾ�ĸ÷�Ӧ����v(CO2)=____���������µ�ƽ�ⳣ��K��____��
(2) ��15 min���¶ȵ�����T2�����ݱ仯���ϱ���ʾ����T1___T2(��������������������=�� )��
(3)��30 minʱ������T2���䣬����������ټ�������ַ�Ӧ������2 mol����ÿ��淴Ӧ���մ�ƽ��ʱNO��ת������=_______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com