
����������SnSO4�������Ȼ�����SnCl4��������ӡȾ�͵�ƹ�ҵ��
��1��ij�о�С�����SnSO4�Ʊ�·�����£�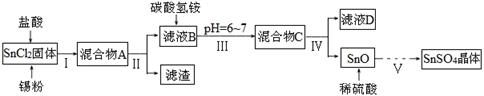
��֪�����������£�����ˮ��Һ����Sn2����Sn4��������Ҫ������ʽ��Sn2���ױ�������SnCl2����ˮ�⡣
��SnCl2���ܺ����Sn�۵������� ��
�ڲ�������õ��IJ����������ձ���� �����������Ҫϴ�ӹ���SnO�к��е����ʣ�����SnO�е�Cl���Ƿ�ϴ�Ӹɾ��IJ���Ϊ ��
�۲�����漰���IJ����У�a.���� b.ϴ�� c.����Ũ�� d.��ȴ�ᾧ e.���¸��������ȷ�IJ���˳��Ϊ ��
��2��ʵ������������װ�ã������ڵĽ���������﴿����������ȡ��ˮSnCl4��SnCl4�۵㣭33�棬�е�114.1�棬����ʪ��������ˮ�⣩���˷�Ӧ���̷ų��������ȡ�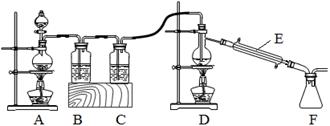
��װ��C��Ӧ�����Լ�Ϊ___________������E������Ϊ____________________��
�ڷ�Ӧ��ʼ����SnCl4ʱ��������Ϩ��___������ĸ��ţ����ľƾ��ƣ�������________��
�۸�ʵ��װ������д���ȱ�ݣ��Ľ��ķ����ǣ������������Լ�������λ�õȣ�______________��
��1���ٷ�ֹSn2����������2�֣� ����ͨ©������©��������������2�֣���ȡ���һ��ϴ��Һ�������Թ��У��μ�ϡ�����AgNO3��Һ��������������֤��SnO�е�Cl����ϴ�Ӹɾ�����������ɫ����֤��SnO�е�Cl��δϴ�Ӹɾ���3�֣� ��cdabe��2�֣�
��2����Ũ���ᣨ2�֣��������ܣ�2�֣�
��D��2�֣������ڵĽ�������������Ӧ�����зų��������ȣ���ά�ָ÷�Ӧ�������У�2�֣�
����Ҫ�¶ȼƣ���װ��F��Ӧ����һ��װ�м�ʯ�ң����������ƹ��壩�ĸ���ܣ���U�ܣ���2�֣�
���������������1��������Sn2���ױ�����������SnCl2���ܺ����Sn�۵������Ƿ�ֹSn2����������
�ڲ�����еõ�©ҺB��������˵���ò����ǹ��ˣ�����õ��IJ����������ձ������ͨ©������©�������������������ӵļ���һ�����������ữ����������Һ�����Լ���SnO�е�Cl���Ƿ�ϴ�Ӹɾ��IJ���Ϊ��ȡ���һ��ϴ��Һ�������Թ��У��μ�ϡ�����AgNO3��Һ��������������֤��SnO�е�Cl����ϴ�Ӹɾ�����������ɫ����֤��SnO�е�Cl��δϴ�Ӹɾ���
�۴���Һ�еõ��������ȷ����������Ũ����.��ȴ�ᾧ�����ˡ�ϴ�ӡ����¸�������ȷ��˳����cdabe��
��2��������SnCl4�۵㣭33�棬�е�114.1�棬����ʪ��������ˮ�⣬���ͨ������������Ǹ���ģ���װ��C��Ӧ�����Լ�ΪŨ������������Ľṹ�ص��֪������E������Ϊ�����ܡ�
���������ڵĽ�������������Ӧ�����зų��������ȣ���ά�ָ÷�Ӧ�������У����Է�Ӧ��ʼ����SnCl4ʱ��������Ϩ��D���ľƾ��ơ�
�۷�Ӧ�������һ���¶ȷ�Χ֮�ڣ���Ҫ�¶ȼƣ���������֪SnCl4����ʪ�����㷢��ˮ�ⷴӦ��������Ӧ������Ӧ��װ��F��Ӧ����һ��װ�м�ʯ�ң����������ƹ��壩�ĸ���ܣ���U�ܣ��ɴﵽĿ�ġ�
���㣺���������Ʊ���ʵ�鷽������������Լ���ѧʵ������������й��ж�


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��������ʵ���ó�����Ӧ������ȷ����
| | ʵ����ʵ | ���� |
| A | �������������ܽ�������������Һ�� | �����������ڼ� |
| B | CO2��ˮ��Һ�ɵ��� | CO2�ǵ���� |
| C | SO2ͨ�����Ը��������Һ����Һ��ɫ | SO2��Ư���� |
| D | ���ֱ����������Ӧ�õ��Ȼ����������� | �����������Դ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��û�ѧ����ش��������⣺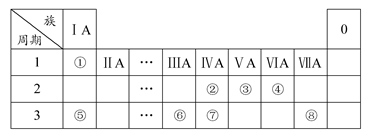
(1)�ܢݢ�ԭ�Ӱ뾶��С�����˳��Ϊ(��Ԫ�ط��ű�ʾ) ��
�ڢۢߵ���ۺ����������������ǿ��˳����(�û�ѧʽ��ʾ) ��
(2)д��������ijЩԪ�ع��ɵļȺ����Ӽ����ֺ����Թ��ۼ��Ļ�����ĵ���ʽ(д��һ�ּ���) ��
(3)��֪������1 g�ٵ����ڢܵ�����ȼ�������ȶ���Һ̬����ʱ�ų�142.9 kJ���������ʾ�ٵ���ȼ�յ��Ȼ�ѧ����ʽΪ ��
(4)�٢��γɵ���Ļ�������ܵ�����KOH���������Һʱ���γ�ԭ��أ����и����ĵ缫��ӦΪ ��
(5)�ɱ���Ԫ�آ٢ۢܢޢ����γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��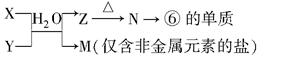
��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ ��
��N���ĵ��������õķ����� ��
��M��Һ�����ԣ�ԭ����(�����ӷ���ʽ����) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪X��Y��Z��M��Q��G��R��T��ǰ�����ڵ�8��Ԫ�أ�δ����ԭ���������У���ZԪ�ص�ij���⻯������������ȼ�ϣ�QԪ�صĵ���Ϊ��ɫֲ�������õ�һ�ֲ�����ݻ�ѧ�Խ��߷���GԪ�ضԽ��ߵ���һ��Ԫ��T�ĵ��ʿ�����RԪ���������������Ӧˮ��Һ�в���Ŀǰ�����ް���GԪ����ࣻYԪ�صļ۵����Ų�ʽΪ��n+1��Sn ��n+1��P��n+3���� XԪ��ij�ֵ�����Һ������MԪ�صĵ��ʷ�����Ӧ����������XԪ�صĵ��ʣ������ߺ˵�������3����MԪ�صĵ����ǵؿǺ����ڶ��ߵĽ���Ԫ�أ�RԪ��Ϊ�������ڵ�ij��Ԫ�ء�
��1��д��XԪ�صļ۵����Ų�ʽ ��ZԪ�ض�Ӧ������⻯��Ŀռ乹�� ��
��2��д��Ԫ��T�ĵ��ʿ�����RԪ���������������Ӧˮ��Һ��Ӧ�����ӷ���ʽ��
��
��3��QԪ������Ӧ���⻯��ķе��ͬ����Ԫ������Ӧ�⻯��е�ߵ�ԭ���ǣ�
��
��4�������У�Ӧ����Y��QԪ�ص�ij�ַ�ĩ״�����ܷ⡢�ܹ⡢���ﱣ�棬�Ҹ÷�ĩ״���ʾ�����ϴ�·�ʱ�õ������û�ѧ����ʽ˵���������ʳ����ڿ���ʧЧ��ԭ��
��
��5��ZԪ�ص�ij���⻯����������ȼ�ϣ��Ҹû�������Zԭ������ԭ�ӵĸ�����Ϊ1��2����ҵ�������غ�Ư��Һ����Ҫ��Ч�ɷ֣���RԪ���������������Ӧˮ��Һ�в��ø��������Ϊ������Ӧ����������Ϊˮ�ϰ��Ļ�������ɵ�����������ij�����ӣ�������CaCl2��Һ��Ӧ����ij�ְ�ɫ����������ʯ����Ҫ�����ֳɷ֡�ͨ���������ϣ�д���û�ѧ����ʽ��
��
��6����ȡMԪ�ص�ij�����������Թ��У�����ϡ�����pHԼΪ7���������-KI��Һ��QԪ�ص�ij�ֻ�����Ҹû�����Ŀռ乹��Ϊ���������Σ���Ӧ�����Һ����ɫ���к��ɫ�������ɡ�������2mol I-ʱ����ת��3mol���ӣ��÷�Ӧ�����ӷ���ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
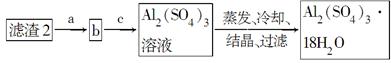
��ij�����Ļ����(��65%Cu��25%Al��8%Fe������Au��Pt)�Ʊ�����ͭ�������������·��������£�
��֪���ʿ�ʼ�����ͳ�����ȫʱpH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Cu(OH)2 |
| ������ʼʱpH | 2.7 | 4.1 | 8.3 |
| ������ȫʱpH | 3.7 | 5.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ����գ�
��1����ȥNaHCO3��Һ�е�����Na2CO3�������ǣ� ��
��2��������������õ�ҩƷ�� ����Ӧ�Ļ�ѧ����ʽΪ�� ��
��3����������ȡ�������������õ��Լ�Ϊ �����ӷ���ʽΪ�� ��
��4��д����������Һ�ڿ����б��ʵĻ�ѧ����ʽ�� ��
��5��д������ʯ������������Ư�۵Ļ�ѧ����ʽ�� ��
��6����CaMg3Si4O12��дΪ���������ʽ��______________________________________________��
��7��д��ʵ������ȡ�����Ļ�ѧ����ʽ_____________________________����Ӧת�Ƶ�������Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��Q��R�����ֶ�����Ԫ�أ�ԭ��������������X��Y��Ԫ����������������֮�;�Ϊ0��Q��Xͬ���壻Z��R�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء���ش��������⣺
��1������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳���ǣ�дԪ�ط��ţ� ��
��2��X��Y���γɶ��ֻ�������мȺ����Լ��ֺ��Ǽ��Լ�������Է���������С�����ʣ�д����ʽ�� ��
��3��������ijЩԪ����ɵĻ�����A��B��C��D������ת����ϵ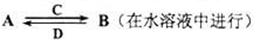
����C������ˮ�����Ե����壻D�ǵ���ɫ���塣д��C�Ľṹʽ ��D�ĵ���ʽ ��
�����A��B��������Ԫ����ɣ�BΪ���Բ������A�Ļ�ѧʽΪ ��
��A�������C��Ӧת��ΪB�����ӷ���ʽ ��
�����A������Ԫ����ɣ�B������Ԫ����ɣ�A��B��Һ���Լ��ԡ������ӷ���ʽ��ʾA��Һ�Լ��Ե�ԭ�� ��A��BŨ�Ⱦ�Ϊ0��1mol/L�Ļ����Һ�У�����Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | �� | �� | |
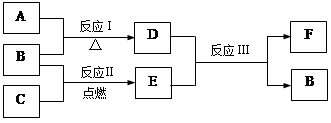
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com