
����Ŀ����.ʵ����Ҫ����500 mL 0.2 mol/L NaOH��Һ����ش��������⣺
��1�����ƹ����в���Ҫʹ�õĻ�ѧ������________������ĸ����
A �ձ� B 500 mL����ƿ C ©�� D ��ͷ�ι� E ������
��2����������ƽ��ȡ�������ƣ�������Ϊ________ g��
��3��������Ҫ�����������ȷ˳����________������ţ���
�ٳ�ȡһ���������������ƣ������ձ��У�����������ˮ�ܽ⣻
�ڼ�ˮ��Һ��������ƿƿ���̶�����1��2 cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�۴���ȴ�����º���Һת�Ƶ�500 mL����ƿ�У�
�ܸǺ�ƿ�����������µߵ���ҡ�ȣ�
������������ˮϴ���ձ��ڱںͲ�����2��3�Σ�ϴ��Һת�Ƶ�����ƿ�С�
��4�����ʵ�������ȱ�ٲ���ݣ���ʹ���Ƴ���NaOH��ҺŨ��_______������ƫ�ߡ�ƫ������������������
��.��ͼʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
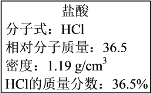
��1����Ũ������HCl�����ʵ���Ũ��Ϊ______ mol/L��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����______��
A ��Һ��HCl�����ʵ������� B ��Һ��Ũ��
C ��Һ��Cl������Ŀ D ��Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.400 mol/L��ϡ���ᡣ��ѧ����Ҫ��ȡ______mL����Ũ����������ơ�
��.����0.27Kg��������Ϊ10����CuCl2��Һ,����Һ��CuCl2�����ʵ���Ϊ___________
���𰸡�C 4.0g �٢ۢݢڢ� ƫ�� 11.9 BD 16.8 0.2
��������
��.��1����Һ���ƹ�������Ҫ�ձ��ܽ���壬��Ҫ500mL����ƿ������Һ����Ҫ��ͷ�ι����ݣ���Ҫ���������������������û���õ�����©������ѡC��
��2����������ƽ��ȡ�������ƣ�������Ϊm=c��V��M=0.2mol/L��0.5L��40g/mol=4.0g���ʴ�Ϊ��4.0g��
��3��ʵ������IJ��裺���㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������������������˳��Ϊ���٢ۢݢڢܣ��ʴ�Ϊ���٢ۢݢڢܣ�
��4����ϴ��Һ�к������ʣ�δ��ϴ��Һת������ƿ�����ʵ��������٣�Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��.��1������������Ϊ1L�������ʵ�����Ϊ1000mL��1.19g cm-3��36.5%�����ʵ����ʵ���Ϊ![]() =11.9mol���������ʵ����ʵ���Ũ��Ϊ
=11.9mol���������ʵ����ʵ���Ũ��Ϊ![]() =11.9mol/L���ʴ�Ϊ��11.9��
=11.9mol/L���ʴ�Ϊ��11.9��
��2��A.n=cV����������Һ����йأ���A����B.��Һ��Ũ���Ǿ�һ�ȶ��ģ�����ȡ��Һ������أ���B��ȷ��C.N=nNA=cVNA����������Һ����йأ���C���� D.��Һ���ܶ��Ǿ�һ�ģ���������ȡ��Һ������أ���D��ȷ����ѡ��BD��
��3��������Һϡ��ǰ�����ʵ��������֪��V(Ũ����)��11.9=0.400mol��L��1��0.5L��V(Ũ����)= 0.0168L=16.8mL������������������ǣ�16.8��
��. ����������������������Ȼ�ͭ��Һ�к����Ȼ�ͭ������Ϊ��0.27Kg��0.1=0.027Kg����Ϊ27g�Ȼ�ͭ��Һ����������ʵ���Ϊ![]() =0.2mol��
=0.2mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����ʾװ��ͨ��10 min����ֱ����Դ�����ӳ�ͼ����ʾװ�ã��ɹ۲쵽U�ι�������缫���������˰�ɫ��״���ʣ�U�ι��Ҳ�Һ������������˵����ȷ����( )

A. ͬ��ͬѹ�£�װ�â���ʯī�缫�Ϸ��õ�����������缫�Ϸ��õ��������
B. װ�â������缫�ĵ缫��ӦʽΪFe��2e����2OH��===Fe(OH)2��
C. װ�â���ʯī�缫�ĵ缫��ӦʽΪ2H����2e��===H2��
D. װ�â�ͨ��10 min�����缫������Һ��pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���õ�ⷨ������Cr2O72���ķ�ˮ��̽����ͬ���ضԺ�Cr2O72����ˮ������Ӱ�죬��������ʾ(Cr2O72������ʼŨ�ȡ��������ѹ�����ʱ�����ͬ)������˵���������
ʵ�� | i | ii | iii | iv |
|
�Ƿ����Fe2(SO4)3 | �� | �� | ����30g | �� | |
�Ƿ����H2SO4 | �� | ����1mL | ����1mL | ����1mL | |
�������� | ʯī | ʯī | ʯī | ʯī | |
�������� | ʯī | ʯī | ʯī | �� | |
Cr2O72����ȥ���� | 0.092% | 12.7% | 20.8% | 57.3% | ʵ��iii��Fe3+ȥ��Cr2O72���Ļ��� |
A. ʵ�颢��ʵ��i�Աȣ������������䣬����c(H+)������Cr2O72����ȥ��
B. ʵ�颣��ʵ�颢�Աȣ������������䣬����c(Fe3+)������Cr2O72����ȥ��
C. ʵ�颤��Fe2+ѭ�����������Cr2O72����ȥ����
D. ��ʵ�颤��ȥ��0.01 mol Cr2O72�������ɵ�������ȫ��ת���ɳ������������������2.06g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�е�ÿһ�������ʾ�йص�һ�ַ�Ӧ������������A��CΪ��ɫ����
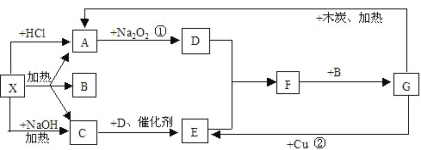
��1��д���й����ʵĻ�ѧʽX��_______��F_______
��2��д��A��D�Ļ�ѧ����ʽ____________����
��3��д��ʵ�����Ʊ�C�Ļ�ѧ����ʽ_______
��4��C�������Ʊ����أ�����CO(NH2)2�����ڸ��������������������ط���ˮ�⣬�����������壬��ˮ��Ļ�ѧ����ʽ��______
��5���ֱ�ȡ����50mLNaOH��Һ����������ͨ��һ����������A������ȡ��Һ10mL�ֱ���ϡ�͵���ͬ������õ���Һ���ң��ֱ����������μ���0.1mol/L��HCl��Һ��������A�����������״���£����������HCl�����֮��Ĺ�ϵ��ͼ��ʾ����
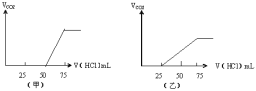
��NaOH������A�������ͼ��ʾ��Һ�д��ڵ������ǣ�_____�������ʵ���֮���ǣ�_________
��ԭNaOH��Һ�����ʵ���Ũ����_______mol/L����ͼ��ʾ��Һ��������A���Ϊ_____mL(��״��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�ܱ������з������·�Ӧ��X(g)��3Y(g)![]() 2Z(g)����H��0����ͼ��ʾ�÷�Ӧ�����ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��t2��t3��t5ʱ��������������ı䣬����û�иı�����ʵij�ʼ������������˵������ȷ����
2Z(g)����H��0����ͼ��ʾ�÷�Ӧ�����ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��t2��t3��t5ʱ��������������ı䣬����û�иı�����ʵij�ʼ������������˵������ȷ����

A. t2ʱ�����˴��� B. t3ʱ�������¶�
C. t5ʱ������ѹǿ D. t4��t5ʱ����ת����һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��д������ʽ��
��1������ϡ���ᣨд�����ӷ���ʽ��___��
��2������������Һ��ϡ���ᣨд�����ӷ���ʽ��__��
��3������ͭ��ϡ���ᣨд�����ӷ���ʽ��__��
��4���Ʊ������������壨д����Ӧ�Ļ�ѧ����ʽ��___��
��5��CO32-+2H+=CO2��+H2O��д����Ӧ�Ļ�ѧ����ʽ��__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ᴿ��������ʵķ����У�A����ȡ��Һ B�����ȷֽ� C�������ᾧ D����Һ E������ F������ G�������ȣ��뽫�ᴿ������������ں�������ϡ�
��1�����뱥��ʳ��ˮ����ɳ�Ļ����___��
��2������Fe(OH)3����![]() ����NaCl��Һ
����NaCl��Һ![]() ___��
___��
��3���������ܵ�CCl4(�е�Ϊ76.75��)�ͼױ�(110.6��)�Ļ����___��
��4���ӵ�ˮ����ȡ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵������ȷ����
A. ��0. 1 mol Na2SiO3����Һ�еμ����ᣬ���ɵ�H2SiO3�����н�������ĿΪ0.1NA
B. ���³�ѹ�£�1.8g��(��CD3)�к��е�������ΪNA
C. �����£�1L pH=10�İ�ˮ��Һ�У����������ˮ������Ϊ1��10-10 NA
D. 7.8gNa2O2�ֱ��������CO2������SO2��ַ�Ӧ��ת�Ƶĵ�����Ŀ��Ϊ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ͨ����MnO2��Ũ���ᷴӦ��ȡ�������䷴Ӧ�Ļ�ѧ����ʽΪ��MnO2 + 4HCl(Ũ) ![]() MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O
��1��������������ʾ�÷�Ӧ����ת�Ƶķ������Ŀ��___________��
��2���ڸ÷�Ӧ�У�����1 mol Cl2���ɣ���������HCl�����ʵ�����___________��ת�Ƶ��ӵ���Ŀ��_____________��
��3��ij�¶��£���Cl2ͨ��NaOH��Һ�У���Ӧ�õ�����ClO-��ClO3-���ʵ���֮��Ϊ1��1�Ļ��Һ����Ӧ�Ļ�ѧ����ʽ�� _________________________ ��
��4����ֽ�����˶�����������ϴʱ������ʹ�á�����顱����Ҫ�ɷ������ᣩ�롰84����Һ������Ҫ�ɷ���NaClO�����������ж����¼����Ը�����Ļ�ѧ֪ʶ������ԭ���ǣ������ӷ���ʽ��ʾ��_________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com