
| ʵ�鲽�� | ʵ����� �����Դ�ǿ������˳���ȡ��塢�� |
| ����ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ | |
| ��NaBr��Һ+��ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ | |
| ��KI��Һ+��ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ |
���� ��1����ʵ������Ͳ��ȡ�Լ��������Թ��н��з�Ӧ��
��2�����з�Ӧ�Ļ�ѧ����ʽΪ 2NaBr+Cl2=2NaCl+Br2�����з�Ӧ�����ӷ���ʽΪ2I-+Cl2=2Cl-+I2��
��3�����Ȼ�̼���μӷ�Ӧ����±�ص��ʲ�������ˮ�����������Ȼ�̼��
��4�����ܱȽ��塢���������ǿ�����Դ������
��� �⣺�ɷǽ������ʼ���û���Ӧ���Ƚϵ��ʵ�������ǿ����֪��Cl2���û���Br2��I2��Br2���û���I2����˵��������Cl2��Br2��I2��
��1����ʵ������Ͳ��ȡ�Լ��������Թ��н��з�Ӧ������Ҫ��ͷ�ιܵμ�Һ�壬�ʴ�Ϊ���Թܡ���ͷ�ιܣ�
��2�����з�Ӧ�Ļ�ѧ����ʽΪ 2NaBr+Cl2=2NaCl+Br2�����з�Ӧ�����ӷ���ʽΪ2I-+Cl2=2Cl-+I2���ʴ�Ϊ��2NaBr+Cl2=2NaCl+Br2��2I-+Cl2=2Cl-+I2��
��3�����Ȼ�̼���μӷ�Ӧ����±�ص��ʲ�������ˮ�����������Ȼ�̼������������Ϊ��ȡ����ʹ���ɵ�Br2��I2�������У����ڹ۲����ʴ�Ϊ����ȡ����
��4����Ƶ�ʵ���в���֤�����������ǿ�ڵ⣬�ѵڢ۲���Ϊ��KI��Һ+��ˮ+1mL CCl4�������ã��۲�CCl4����ɫ��
�ʴ�Ϊ��û�бȽ�Br2��I2��������ǿ�����ѵڢ۲���Ϊ������ˮ����KI������ֽ�ϣ��۲���ֽ�Ƿ����ɫ����KI��Һ+��ˮ+1 mL CCl4�������ã��۲����Ȼ�̼����ɫ����
���� ���⿼��±�ص���֮����û���Ӧ��ʵ����ƣ����յ��ʵ�������ǿ��������ķ���Ϊ���Ĺؼ����漰������ԭ��Ӧ�����ԵıȽϼ���ȡ��֪ʶ�㣬��Ŀ�ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
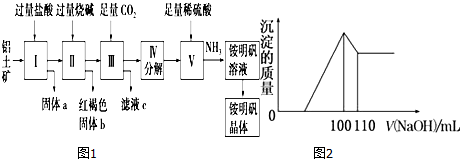
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
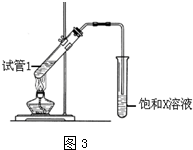
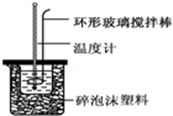 ʵ������50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�����������⣺
ʵ������50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�����������⣺| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�1L������ȫȼ�պ������ɵ���̬����ķ�����Ϊ$\frac{7}{22.4}$NA | |
| B�� | 100ml 0.1mol/LCH3COOH��Һ������п��Ӧ�����ɵ�����������Ϊ0.01NA | |
| C�� | 0.1mol CH4����������ΪNA | |
| D�� | 0.5mol C2H4�к��е�C=C˫����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ��c��H+��Ϊ2 mol•L-1 | B�� | ������������ת�Ƶ�������ΪNA | ||
| C�� | ���õ���Cu�����ʵ���Ϊ1 mol | D�� | ԭ�����Һ�� c��K+��Ϊ5 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
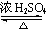 CH3COOCH2CH=CH2+H218O��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽΪ
CH3COOCH2CH=CH2+H218O��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽΪ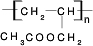 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
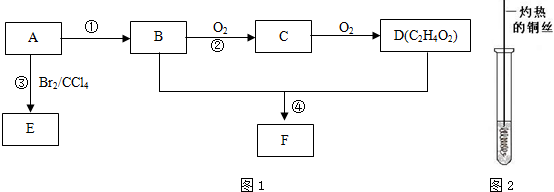
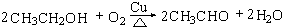 ��
�� ��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪCH3COOH+HCO3-��CH3COO-+H2O+CO2����
��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪCH3COOH+HCO3-��CH3COO-+H2O+CO2�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3 H2O | B�� | CO2 HCl | C�� | NO H2 | D�� | CH4 Br2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com