

���� ��1��a��ú�����л��������������ɵĸ��ӵĻ���
b����ú�к��е�Ԫ�غͲ���ȫȼ�շ�����
c��̼������ȷֽ�Ϊ�����ƣ��������������������������������������Ӧ��
d��ú�ĸ���ú��������Һ�������ڻ�ѧ�仯��
��2��H2S�ð�ˮ��������������狀�ˮ��
��3���ٳ����£�NaHSO3��Һ��pH=6��NaHSO3��Һ�����ԣ�˵������̶ȴ���ˮ��̶ȣ�
������������NaHSO3��Ӧ���ɳ���������ơ�NaOH��ˮ��
��4���پݸ�˹��������֪���Ȼ�ѧ����ʽ������Ӧ����ֵ���мӼ���������Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ����ֵ���мӼ���
��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H��0������Ӧ���������С��ͨ�����������¶Ⱥ�����ѹǿ����ƽ����ƶ�����ͨ��CO��ƽ��ת���ʵı仯�ж�X��Y��
��� �⣺��1��a��ú�����л��������������ɵĸ��ӵĻ�����Ҫ����CԪ�أ���a��ȷ��
b��ú�а�����Ԫ�غ͵�Ԫ�أ�����ȫȼ��ʱ����һ����̼����������������̳����к����ʣ���b��ȷ��
c��̼������ȷֽ�Ϊ�����ƣ�������������������������������ơ������Ʒ�����Ӧ����������ƣ�Ȼ���������������ȶ��������Σ���c��ȷ��
d��ú�ĸ���ú��������Һ�������ڻ�ѧ�仯����d����
�ʴ�Ϊ��abc��
��2��H2S�ð�ˮ��������������狀�ˮ�����ӷ���ʽΪ��H2S+2NH3•H2O�T2NH4++S2-+2H2O��
�ʴ�Ϊ��H2S+2NH3•H2O�T2NH4++S2-+2H2O��
��3���ٳ����£�NaHSO3��Һ��pH=6��NaHSO3��Һ�����ԣ�NaHSO3��Һ�д��������������ˮ������룬ˮ�����ʼ��ԣ����뵼��������ԣ��ݴ˷���������̶ȴ���ˮ��̶ȣ�������Ũ�ȴ�СΪ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
������������NaHSO3��Ӧ���ɳ���������ơ�NaOH��ˮ����ѧ����ʽΪCa��OH��2+NaHSO3�TCaSO3��+NaOH+H2O��
�ʴ�Ϊ��Ca��OH��2+NaHSO3�TCaSO3��+NaOH+H2O��
��4���٣���2H2��g��+CO��g��?CH3OH��g����H=-90.8kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.3kJ•mol-1
�ɸ�˹���ɢ�+��+�١�2�õ�3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H=-246.4 kJ•mol-1��
�ʴ�Ϊ��-246.4 kJ•mol-1��
��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H��0������Ӧ���������С�������¶ȣ�ƽ�������ƶ���CO��ƽ��ת���ʼ�С������ѹǿ��ƽ�������ƶ���
CO��ƽ��ת�����������X����ѹǿ��Y�����¶ȣ���ѹǿ�����£��¶�Խ�ߣ�CO��ƽ��ת����ԽС�����Y1��Y2��
�ʴ�Ϊ��Y1��Y2����3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����֪��ѹǿ����CO��ƽ��ת���������¶����ߣ�CO��ƽ��ת���ʼ�С������X����ѹǿ��Y�����¶ȣ�ѹǿһ��ʱ���¶�Խ��ƽ��ת����Խ��
���� ���⿼���Ϊ�ۺϣ��漰ú���ۺ����á���ѧ�����ӣ�����ʽ����д������Ũ�ȵĴ�С�Ƚϡ���Ӧ�ȵļ���ͻ�ѧƽ����ƶ�����Ŀ�Ѷ��еȣ����յ���ƽ�⡢ˮ��ƽ��Ȼ�ѧƽ�⼰������Ϊ���Ĺؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{1}{14}$mol/L | B�� | $\frac{4}{5}$mol/L | C�� | $\frac{1}{28}$mol/L | D�� | $\frac{1}{42}$mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=3�������У���c��H+����pH=1�������е�3�� | |
| B�� | 1mol/L0.5L�� AlCl3��Һ�У������ӵ����ʵ�������0.5mol | |
| C�� | ͬ�¶�ͬ���ʵ���Ũ��ʱ��HF��HCN���룬��NaF��Һ�� pH �� NaCN ��Һ�� | |
| D�� | ����ˮ�������c��H+��=1��10-12mol/L����Һ�У�K+��ClO-��SO42-һ���ܴ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��I-��CO${\;}_{3}^{2-}$��ClO- | B�� | Fe2+��H+��K+��NO${\;}_{3}^{-}$ | ||
| C�� | Ba2+��Na+��SCN-��Cl- | D�� | Cu2+��Fe2+��Cl-��NO${\;}_{3}^{-}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� ���� | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� | �� |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��һ����ǿ�� | B�� | ��һ�������� | C�� | ��һ���Ƕ�Ԫ�� | D�� | ��һ����һԪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �� ��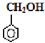 | B�� | ���͡� | ||
| C�� | CH3COCH3 �� CH3CHO | D�� | CH3Cl ��ClCH2CH2Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
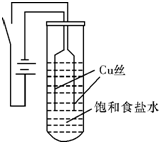
| ���� | �Ȼ�ͭ | ������ͭ | ��������ͭ �����ȶ��� | �Ȼ���ͭ |
| ��ɫ | �������ɫ��Ũ��Һ����ɫ��ϡ��Һ����ɫ | ��ɫ | �Ȼ�ɫ | ��ɫ |
| A�� | ���������������������ƶ� | B�� | ��ʼʱ����Cu �ŵ�����Cu2+ | ||
| C�� | ������ӦΪ2H2O+2e-=H2��+2OH- | D�� | ����ҺpH����CuCl��ת��ΪCuOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ʵ�鲽�� | ʵ����� �����Դ�ǿ������˳���ȡ��塢�� |
| ����ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ | |
| ��NaBr��Һ+��ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ | |
| ��KI��Һ+��ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com