
ЁОЬтФПЁПЯТСаШШЛЏбЇЗНГЬЪНЃЌе§ШЗЕФЪЧ
A.вбжЊжаКЭШШЮЊ57.3 kJ/molЃКCH3COOH(aq)ЃЋNaOH(aq)ЃНCH3COONa(aq)ЃЋH2O(l) ІЄHЃНЃ57.3 kJ/mol
B.1 mol SO2гы2 mol O2дкФГУмБеШнЦїжаЗДгІЗХГі88 kJШШСПЃЌдђЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊЃК2SO2(g)ЃЋO2(g)![]() 2SO3(g) ЁїHЃНЃ176 kJ/mol
2SO3(g) ЁїHЃНЃ176 kJ/mol
C.МзЭщЕФШМЩеШШЮЊ890.3kJЁЄmolЃ1ЃЌдђМзЭщШМЩеЕФШШЛЏбЇЗНГЬЪНПЩБэЪОЮЊЃКCH4(g)ЃЋ2O2(g)ЃНCO2(g)ЃЋ2H2O(g) ІЄHЃНЃ890.3 kJЁЄmolЃ1
D.8 g ЙЬЬхСђЭъШЋШМЩеЩњГЩSO2ЃЌЗХГі74kJШШСПЃКS(s)ЃЋO2(g)ЃНSO2(g) ІЄHЃНЃ296 kJ/mol
ЁОД№АИЁПD
ЁОНтЮіЁП
A.CH3COOHЪЧШѕЫсЃЌЕчРыашЮќШШЃЌЙЪAДэЃЛ
B.2SO2(g)+O2(g) ![]() 2SO3(g) ЪЧПЩФцЗДгІЃЌЫљвд1molSO2гы2moO2дкФГУмБеШнЦїжаЗДгІЯћКФSO2ЕФЮяжЪЕФСПаЁгк1molЃЌЖјЗДгІШШгІЮЊЯћКФ2molSO2ЫљЗХГіЕФШШСПЃЌЙЪBДэЃЛ
2SO3(g) ЪЧПЩФцЗДгІЃЌЫљвд1molSO2гы2moO2дкФГУмБеШнЦїжаЗДгІЯћКФSO2ЕФЮяжЪЕФСПаЁгк1molЃЌЖјЗДгІШШгІЮЊЯћКФ2molSO2ЫљЗХГіЕФШШСПЃЌЙЪBДэЃЛ
C.МзЭщЕФШМЩеШШЪЧжИ1molМзЭщЭъШЋШМЩеЩњГЩCO2(g)ЁЂ H2O(l)ЫљЗХГіЕФШШСПЃЌЙЪCДэЃЛ
D.8gСђЮяжЪЕФСП=![]() ЃЌЭъШЋШМЩеЩњГЩSO2ЗХГі74kJШШСПЃЌ1molСђШМЩеЗХГіЕФШШСПЪЧ
ЃЌЭъШЋШМЩеЩњГЩSO2ЗХГі74kJШШСПЃЌ1molСђШМЩеЗХГіЕФШШСПЪЧ![]() kJ =296kJЃЌШШЛЏбЇЗНГЬЪНЮЊS(s)ЃЋO2(g)ЃНSO2(g) ІЄHЃНЃ296 kJ/molЃЌЙЪDе§ШЗЃЛ
kJ =296kJЃЌШШЛЏбЇЗНГЬЪНЮЊS(s)ЃЋO2(g)ЃНSO2(g) ІЄHЃНЃ296 kJ/molЃЌЙЪDе§ШЗЃЛ
ЙЪбЁЃКDЁЃ


 ЛЅЖЏПЮЬУЯЕСаД№АИ
ЛЅЖЏПЮЬУЯЕСаД№АИ МЄЛюЫМЮЌжЧФмбЕСЗПЮЪБЕМбЇСЗЯЕСаД№АИ
МЄЛюЫМЮЌжЧФмбЕСЗПЮЪБЕМбЇСЗЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПздШЛНчжаДцдкДѓСПЕФН№ЪєдЊЫиЃЌЦфжаФЦЁЂУОЁЂТСЁЂЬњЁЂЭЕШдкЙЄХЉвЕЩњВњжагазХЙуЗКЕФгІгУЁЃЛиД№ЯТСаЮЪЬт:
(1)CuSO4КЭCu(NO3)2жабєРызгКЫЭтЕчзгХХВМЪНЮЊ__________ЃЌNЁЂOЁЂSдЊЫиЕФдзгЖдМќКЯЕчзгЮќв§СІзюДѓЕФЪЧ___________ЁЃ
(2)дкСђЫсЭШмвКжаМгШыЙ§СПKCNФмЩњГЩХфРызг[Cu(CN)4]2-ЃЌ1mol CNжаКЌгаЕФІаМќЕФЪ§ФПЮЊ__________ЁЃгыCNЛЅЮЊЕШЕчзгЬхЕФРызгЛђЗжзгга__________(аДГівЛжжМДПЩ)ЁЃ
(3)[Cu(NH3)4]2+жаЃЌЬсЙЉЙТЖдЕчзгЕФЪЧ___________ЁЃCu(NH3)2Cl2гаСНжжЭЌЗжвьЙЙЬхЃЌЦфжавЛжжПЩШмгкЫЎЃЌдђДЫжжЛЏКЯЮяЪЧ___________(ЬюЁАМЋадЁБЛђЁАЗЧМЋадЁБ)ЗжзгЃЌгЩДЫЭЦжЊ[Cu(NH)4]2+ЕФПеМфЙЙаЭЪЧ___________ЁЃЃЈЬюЁАЦНУце§ЗНаЮЁБЛђЁАе§ЫФУцЬхЁБЃЉ
(4)NH3жаNдзгЕФдгЛЏЗНЪНЪЧ___________ЃЌСђдЊЫиЖдгІЕФКЌбѕЫсЫсадЪЧH2SO4ЧПгкH2SO3ЃЌЦфдвђЮЊ___________ЁЃ
(5)ЭЕФвЛжжбѕЛЏЮяОЇЬхНсЙЙШчЭМЫљЪОЃЌИУбѕЛЏЮяЕФЛЏбЇЪНЪЧ___________ЁЃШєИУОЇЬхНсЙЙЮЊГЄЗНЬхЃЌЦфВЮЪ§ШчЭМЃЌАЂЗќМгЕТТоГЃЪ§ЮЊNAЃЌдђИУбѕЛЏЮяЕФУмЖШЮЊ___________g/cm3ЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖдгкПЩФцЗДгІЃК2SO2ЃЋO2![]() 2SO3ЃЌЁїHЃМ0ЯТСаДыЪЉФмЪЙЗДгІЮяжаЛюЛЏЗжзгАйЗжЪ§ЁЂЛЏбЇЦНКтзДЬЌЖМЗЂЩњБфЛЏЕФЪЧ(ЁЁЁЁ)
2SO3ЃЌЁїHЃМ0ЯТСаДыЪЉФмЪЙЗДгІЮяжаЛюЛЏЗжзгАйЗжЪ§ЁЂЛЏбЇЦНКтзДЬЌЖМЗЂЩњБфЛЏЕФЪЧ(ЁЁЁЁ)
A. діДѓбЙЧП B. Щ§ИпЮТЖШ C. ЪЙгУДпЛЏМС D. ЖрГфO2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПбаОПЗЂЯжЃЌNOxКЭSO2ЪЧЮэіВЕФжївЊГЩЗжЁЃ
Ђё. NOxжївЊРДдДгкЦћГЕЮВЦјЃЌПЩвдРћгУЛЏбЇЗНЗЈНЋЖўепзЊЛЏЮЊЮоЖОЮоКІЕФЮяжЪЁЃ
вбжЊЃКN2(g)ЃЋO2(g) ![]() 2NO(g)ЁЁІЄHЃНЃЋ180 kJЁЄmolЃ1
2NO(g)ЁЁІЄHЃНЃЋ180 kJЁЄmolЃ1
2CO(g)ЃЋO2(g) ![]() 2CO2(g)ЁЁІЄHЃНЃ564 kJЁЄmolЃ1
2CO2(g)ЁЁІЄHЃНЃ564 kJЁЄmolЃ1
ЃЈ1ЃЉ2NO(g)ЃЋ2CO(g)![]() 2CO2(g)ЃЋN2(g)ЁЁІЄHЃН________.
2CO2(g)ЃЋN2(g)ЁЁІЄHЃН________.
ЃЈ2ЃЉTЁцЪБЃЌНЋЕШЮяжЪЕФСПЕФNOКЭCOГфШыШнЛ§ЮЊ2 LЕФУмБеШнЦїжаЃЌБЃГжЮТЖШКЭЬхЛ§ВЛБфЃЌЗДгІЙ§ГЬ(0ЁЋ15 min)жаNOЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЭМЫљЪОЁЃ

ЂйвбжЊЃКЦНКтЪБЦјЬхЕФЗжбЙЃНЦјЬхЕФЬхЛ§ЗжЪ§ЁСЬхЯЕЕФзмбЙЧПЃЌTЁцЪБДяЕНЦНКтЃЌДЫЪБЬхЯЕЕФзмбЙЧПЮЊp=20MPaЃЌдђTЁцЪБИУЗДгІЕФбЙСІЦНКтГЃЪ§Kp ЃН_______ЃЛЦНКтКѓЃЌШєБЃГжЮТЖШВЛБфЃЌдйЯђШнЦїжаГфШыNOКЭCO2Иї0.3molЃЌЦНКтНЋ_____ (ЬюЁАЯђзѓЁБЁЂЁАЯђгвЁБЛђЁАВЛЁБ)вЦЖЏЁЃ
Ђк15 minЪБЃЌШєИФБфЭтНчЗДгІЬѕМўЃЌЕМжТn(NO)ЗЂЩњШчЩЯЭМЫљЪОЕФБфЛЏЃЌдђИФБфЕФЬѕМўПЩФмЪЧ_____(ЬюађКХ)
A.діДѓCOХЈЖШ B.Щ§ЮТ C.МѕаЁШнЦїЬхЛ§ D.МгШыДпЛЏМС
Ђђ. SO2жївЊРДдДгкУКЕФШМЩеЁЃШМЩебЬЦјЕФЭбСђМѕХХЪЧМѕЩйДѓЦјжаКЌСђЛЏКЯЮяЮлШОЕФЙиМќЁЃ
вбжЊЃКбЧСђЫсЃКKa1=2.0ЁС10-2 Ka2=6.0ЁС10-7
ЃЈ3ЃЉЧыЭЈЙ§МЦЫужЄУїЃЌNaHSO3ШмвКЯдЫсадЕФдвђЃК_________________________
ЃЈ4ЃЉШчЭМЪОЕФЕчНтзАжУЃЌПЩНЋЮэіВжаЕФNOЁЂSO2зЊЛЏЮЊСђЫсяЇЃЌДгЖјЪЕЯжЗЯЦјЕФЛиЪедйРћгУЁЃЭЈШыNOЕФЕчМЋЗДгІЪНЮЊ____________________ЃЛШєЭЈШыЕФNOЬхЛ§ЮЊ4.48L(БъПіЯТ)ЃЌдђСэЭтвЛИіЕчМЋЭЈШыЕФSO2жЪСПжСЩйЮЊ________gЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПXЁЂYЁЂZЁЂWЪЧдЊЫижмЦкБэЧАЫФжмЦкжаЕФГЃМћдЊЫиЃЌЦфдзгађЪ§вРДЮдіДѓЁЃXдЊЫиЕФвЛжжКЫЫиЕФжЪСПЪ§ЮЊ12ЃЌжазгЪ§ЮЊ6ЃЛYдЊЫиЪЧЖЏжВЮяЩњГЄВЛПЩШБЩйЕФЁЂЙЙГЩЕААзжЪЕФживЊзщГЩдЊЫиЃЛZЕФЛљЬЌдзгКЫЭт9ИідзгЙьЕРЩЯЬюГфСЫЕчзгЧвга2ИіЮДГЩЖдЕчзгЃЌгыXВЛЭЌзхЃЛWЪЧвЛжжГЃМћдЊЫиЃЌПЩвдаЮГЩ3жжбѕЛЏЮяЃЌЦфжавЛжжбѕЛЏЮяЪЧОпгаДХадЕФКкЩЋОЇЬхЁЃ
(1)аДГіЯТСадЊЫиЕФУћГЦ X_______,Y________ЃЌZ__________
(2)XЁЊHМќКЭYЁЊHМќЪєгкМЋадЙВМлМќЃЌЦфжаМЋадНЯЧПЕФЪЧ________(XЁЂYгУдЊЫиЗћКХБэЪО)МќЁЃXЕФЕквЛЕчРыФмБШYЕФ________(ЬюЁАДѓЁБЛђЁАаЁЁБ)ЁЃ
(3)аДГіXЕФЕЅжЪгыZЕФзюИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЕФХЈШмвКЗДгІЕФЛЏбЇЗНГЬЪНЃК________________ЁЃ
(4)WЕФЛљЬЌдзгЕФМлЕчзгХХВМЪНЮЊ____________ЃЛ
(5)YдЊЫиЕФКЫЭтЕчзгЙьЕРБэЪОЪНЮЊ___________ЁЃ
(6)вбжЊвЛжжY4ЗжзгНсЙЙШчЭМЫљЪОЃК
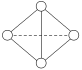
ЖЯСб1 mol YЁЊYМќЮќЪе167 kJЕФШШСПЃЌЩњГЩ1 mol YЁдYЗХГі942 kJШШСПЁЃдђгЩ1molY4ЦјЬЌЗжзгБфГЩ2molY2ЦјЬЌЗжзг_______(ЬюаДЮќЪеЛђЗХГі)_______kJ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЫїЪЯЬсШЁЗЈЪЧВтЖЈЖЏжВЮябљЦЗжаДжжЌЗОКЌСПЕФБъзМЗНЗЈЁЃЦфдРэЪЧРћгУШчЭМзАжУЃЌгУЮоЫЎввУбЕШгаЛњШмМССЌајЁЂЗДИДЁЂЖрДЮнЭШЁЖЏжВЮябљЦЗжаЕФДжжЌЗОЁЃОпЬхВНжшШчЯТЃК

ЂйАќзАЃКШЁТЫжНжЦГЩТЫжНЭВЃЌЗХШыКцЯфжаИЩдяКѓЃЌвЦжСвЧЦїXжаРфШДжСЪвЮТЃЌШЛКѓЗХШыГЦСПЦПжаГЦСПЃЌжЪСПМЧзїa;дкТЫжНЭВжаАќШывЛЖЈжЪСПбаЯИЕФбљЦЗЃЌЗХШыКцЯфжаИЩдяКѓЃЌвЦжСвЧЦїXжаРфШДжСЪвЮТЃЌШЛКѓЗХШыГЦСПЦПжаГЦСПЃЌжЪСПМЧзїbЁЃ
ЂкнЭШЁЃКНЋзАгабљЦЗЕФТЫжНЭВгУГЄФїзгЗХШыГщЬсЭВжаЃЌзЂШывЛЖЈСПЕФЮоЫЎввУбЃЌЪЙТЫжНЭВЭъШЋНўУЛШыввУбжаЃЌНгЭЈРфФ§ЫЎЃЌМгШШВЂЕїНкЮТЖШЃЌЪЙРфФ§ЯТЕЮЕФЮоЫЎввУбГЪСЌжщзДЃЌжСГщЬсЭВжаЕФЮоЫЎввУбгУТЫжНЕуЕЮМьВщЮогЭМЃЮЊжЙ(ДѓдМ6h~12h)ЁЃ
ЂлГЦСПЃКнЭШЁЭъБЯКѓЃЌгУГЄФїзгШЁГіТЫжНЭВЃЌдкЭЈЗчДІЪЙЮоЫЎввУбЛгЗЂЃЌД§ЮоЫЎввУбЛгЗЂКѓЃЌНЋТЫжНЭВЗХШыКцЯфжаИЩдяКѓЃЌвЦжСвЧЦїXжаРфШДжСЪвЮТЃЌШЛКѓЗХШыГЦСПЦПжаГЦСПЃЌжЪСПМЧзїcЁЃ
ЛиД№ЯТСаЮЪЬтЃК
(1)ЪЕбщжаШ§ДЮЪЙгУЕФвЧЦїXЕФУћГЦЮЊ__________________ЁЃЮЊЬсИпввУбеєЦјЕФРфФ§аЇЙћЃЌЫїЪЯЬсШЁЦїПЩбЁгУЯТСа_______(ЬюзжФИ)ДњЁЃ
![]()
![]()
![]()
aЃЎПеЦјРфФ§Йм bЃЎжБаЮРфФ§Йм cЃЎЩпаЮРфФ§Йм
(2)ЪЕбщжаБиаыЪЎЗжзЂвтввУбЕФАВШЋЪЙгУЃЌШчВЛФмгУУїЛ№МгШШЁЂЪвФкБЃГжЭЈЗчЕШЁЃЮЊЗРжЙввУбЛгЗЂЕНПеЦјжааЮГЩШМБЌЃЌГЃдкРфФ§ЙмЩЯПкСЌНгвЛИіЧђаЮИЩдяЙмЃЌЦфжазАШыЕФвЉЦЗЮЊ_______(ЬюзжФИ)ЁЃ
aЃЎЛюадЬП bЃЎМюЪЏЛв cЃЎP2O5 dЃЎХЈСђЫс
ЮоЫЎввУбдкПеЦјжаПЩФмбѕЛЏЩњГЩЩйСПЙ§бѕЛЏЮяЃЌМгШШЪБЗЂЩњБЌеЈЁЃМьбщЮоЫЎввУбжаЪЧЗёКЌгаЙ§бѕЛЏЮяЕФЗНЗЈЪЧ______________________________________ЁЃ
(3)ЪЕбщжаашПижЦЮТЖШдк70Ёц~80ЁцжЎМфЃЌПМТЧЕНАВШЋЕШвђЫиЃЌгІВЩШЁЕФМгШШЗНЪНЪЧ_______ЁЃЕБЮоЫЎввУбМгШШЗаЬкКѓЃЌеєЦјЭЈЙ§ЕМЦјЙмЩЯЩ§ЃЌБЛРфФ§ЮЊвКЬхЕЮШыГщЬсЭВжаЃЌЕБвКУцГЌЙ§ЛиСїЙмзюИпДІЪБЃЌнЭШЁвКМДЛиСїШыЬсШЁЦї(ЩеЦП)жаЁЁИУЙ§ГЬСЌајЁЂЗДИДЁЂЖрДЮНјааЃЌдђнЭШЁвКЛиСїШыЬсШЁЦї(ЩеЦП)ЕФЮяРэЯжЯѓЮЊ_______ЁЃЫїЪЯЬсШЁЗЈгывЛАунЭШЁЗЈЯрБШНЯЃЌЦфгХЕуЮЊ___________________________ЁЃ
(4)Ъ§ОнДІРэЃКбљЦЗжаДПжЌЗОАйЗжКЌСП_________(ЬюЁА<ЁБЁЂЁА>ЁБЛђЁА=ЁБ)(b-c)/(b-a)ЁС100%ЃЛВтЖЈжаЕФбљЦЗЁЂзАжУЁЂввУбЖМашвЊНјааЭбЫЎДІРэЃЌЗёдђЕМжТВтЖЈНсЙћ__________(ЬюЁАЦЋИпЁБЁЂЁАЦЋЕЭЁБЛђЁАЮогАЯьЁБ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТЭМЪЧВПЗжЖЬжмЦкдЊЫиЕФГЃМћЛЏКЯМлгыдзгађЪ§ЕФЙиЯЕЃК
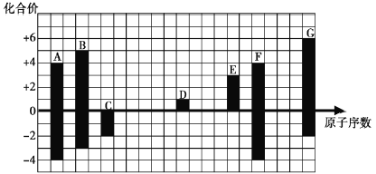
(1)дЊЫиAдкжмЦкБэжаЕФЮЛжУЮЊ________________________ЁЃ
(2)гУЕчзгЪНБэЪОD2GЕФаЮЪНЙ§ГЬЃК_________________________________________________ЃЌЦфЫљКЌЛЏбЇМќРраЭЮЊ__________ЁЃ
(3)C2ЃЁЂDЃЋЁЂG2ЃАыОЖгЩДѓЕНаЁЕФЫГађЪЧ________(ЬюРызгЗћКХ)ЁЃ
(4)CЁЂGЕФМђЕЅЧтЛЏЮяжаЃЌЗаЕуНЯЕЭЕФЪЧ________(ЬюЛЏбЇЪН)ЃЌдвђЪЧ________________ЁЃСНжжЦјЬЌЧтЛЏЮяЕФЮШЖЈадC________G(ЬюЁА>ЁБЛђЁА<ЁБ)ЁЃ
(5)CгыDаЮГЩЕФОпгаЧПбѕЛЏадЁЂПЩвдзіЙЉбѕМСЕФЛЏКЯЮяЕФЕчзгЪНЮЊ_________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯђ5 mL NaClШмвКжаЕЮШывЛЕЮAgNO3ШмвКЃЌГіЯжАзЩЋГСЕэЃЌМЬајЕЮМгвЛЕЮKIШмвКВЂеёЕДЃЌГСЕэБфЮЊЛЦЩЋЃЌдйЕЮШывЛЕЮNa2SШмвКВЂеёЕДЃЌГСЕэгжБфГЩКкЩЋЃЌИљОнЩЯЪіБфЛЏЙ§ГЬЃЌЗжЮіДЫШ§жжГСЕэЮяЕФШмНтЖШЙиЯЕЮЊ
A. AgClЃНAgIЃНAg2S B. AgCl<AgI<Ag2S
C. AgCl>AgI>Ag2S D. AgI>AgCl>Ag2S
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПCl2OФмгыЫЎЗДгІЩњГЩДЮТШЫсЃЌПЩЩБЫРаТаЭЙкзДВЁЖОЕШЖржжВЁЖОЁЃвЛжжжЦБИCl2OЕФдРэЮЊHgO+2Cl2=HgCl2+Cl2OЃЌзАжУШчЭМЫљЪОЁЃ

вбжЊЃКЂйCl2OЕФШлЕуЮЊ-116ЁцЁЂЗаЕуЮЊ3.8ЁцЃЌОпгаЧПСвДЬМЄадЦјЮЖЁЂвзШмгкЫЎЃЛ
ЂкCl2OгыгаЛњЮяЁЂЛЙдМСНгДЅЛђМгШШЪБЛсЗЂЩњШМЩеВЂБЌеЈЁЃ
ЯТСаЫЕЗЈжае§ШЗЕФЪЧЃЈ ЃЉ
A.зАжУЂлжаЪЂзАЕФЪдМСЪЧБЅКЭЪГбЮЫЎ
B.зАжУЂмгыЂнжЎМфПЩгУЯ№НКЙмСЌНг
C.ДгзАжУЂнжавнГіЦјЬхЕФжївЊГЩЗжЪЧCl2O
D.ЭЈШыИЩдяПеЦјЕФФПЕФЪЧНЋЩњГЩЕФCl2OЯЁЪЭЃЌМѕаЁБЌеЈЮЃЯе
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com