
ЁОЬтФПЁПАДвЊЧѓЭъГЩЯТСаМЦЫу
ЃЈ1ЃЉ1mol CO2ЕФжЪСПЮЊ_________ЃЛ
ЃЈ2ЃЉБъзМзДПіЯТЃЌ11.2L NH3КЌгаЕФЗжзгЪ§ЮЊ_________ ЃЛ
ЃЈ3ЃЉ0.1mol/LЕФBaCl2ШмвКжаЃЌCl-ЕФЮяжЪЕФСПХЈЖШЮЊ__________ ЃЛ
ЃЈ4ЃЉБъзМзДПіЯТЃЌФГЦјЬхбѕЛЏЮяЕФЛЏбЇЪНЮЊRO2ЃЌ 1.28 gИУбѕЛЏЮяЕФЬхЛ§ЮЊ448 mLЃЌдђИУбѕЛЏЮяЕФФІЖћжЪСПЮЊ_______ЃЌRЕФЯрЖддзгжЪСПЮЊ________ЃЛ
ЃЈ5ЃЉ18g H2OКЌгаЕФдзгЪ§ЮЊ_________ЃЛЕчзгЪ§ЮЊ________ ЁЃ
ЁОД№АИЁП44g 0.05NA 0.2mol/L 64g/mol 32 3NA 10NA
ЁОНтЮіЁП
ЃЈ1ЃЉ1mol CO2ЕФжЪСПЮЊm=nMЃЛ
ЃЈ2ЃЉБъзМзДПіЯТЃЌ11.2L NH3ЕФЮяРэЕФСПЮЊn= ![]() =
= ![]() =0.05molЃЌЗжзгЪ§ЮЊ0.05NAЃЛ
=0.05molЃЌЗжзгЪ§ЮЊ0.05NAЃЛ
ЃЈ3ЃЉ0.1mol/LЕФBaCl2ШмвКжаЃЌ1ИіBaCl2жагаСНИіCl-ЃЌЙЪCl-ЕФЮяжЪЕФСПХЈЖШЮЊ0.2mol/LЃЛ
ЃЈ4ЃЉБъзМзДПіЯТЃЌ1.28gИУбѕЛЏЮяЕФЬхЛ§ЮЊ448mLЃЌИУЦјЬхЕФЮяжЪЕФСПЮЊЃК![]() =0.02molЃЌИУЦјЬхЕФФІЖћжЪСПЮЊЃК
=0.02molЃЌИУЦјЬхЕФФІЖћжЪСПЮЊЃК![]() =64g/molЃЌЩшRЕФЯрЖддзгжЪСПЮЊxЃЌдђx+16ЁС2=64ЃЌНтЖСx=32ЃЌRЮЊOдЊЫиЃЛ
=64g/molЃЌЩшRЕФЯрЖддзгжЪСПЮЊxЃЌдђx+16ЁС2=64ЃЌНтЖСx=32ЃЌRЮЊOдЊЫиЃЛ
ЃЈ5ЃЉ18gH2OЕФЮяжЪЕФСПЮЊ![]() =1molЃЌ1ИіH2OЗжзгжага3ИідзгЃЌЙЪКЌгадзгЪ§ФПЮЊ1molЁС3NAmol-1=3NAЃЛУПИіH2OЗжзгКЌга10ИіЕчзгЃЌЙЪnЃЈЕчзгЃЉ=1molЁС10=10molЃЌЙЪКЌгаЕчзгЪ§ФПЮЊ10molЁСNAmol-1=10NAЁЃ
=1molЃЌ1ИіH2OЗжзгжага3ИідзгЃЌЙЪКЌгадзгЪ§ФПЮЊ1molЁС3NAmol-1=3NAЃЛУПИіH2OЗжзгКЌга10ИіЕчзгЃЌЙЪnЃЈЕчзгЃЉ=1molЁС10=10molЃЌЙЪКЌгаЕчзгЪ§ФПЮЊ10molЁСNAmol-1=10NAЁЃ
ЃЈ1ЃЉ1mol CO2ЕФжЪСПЮЊm=nM=1molЁС44g/mol=44gЃЌе§ШЗД№АИЪЧ44gЃЛ
ЃЈ2ЃЉБъзМзДПіЯТЃЌ11.2L NH3ЕФЮяРэЕФСПЮЊn= ![]() =
= ![]() =0.05molЃЌЗжзгЪ§ЮЊ0.05NAЃЛ
=0.05molЃЌЗжзгЪ§ЮЊ0.05NAЃЛ
ЃЈ3ЃЉ0.1mol/LЕФBaCl2ШмвКжаЃЌ1ИіBaCl2жагаСНИіCl-ЃЌЙЪCl-ЕФЮяжЪЕФСПХЈЖШЮЊ0.2mol/LЃЛ
ЃЈ4ЃЉБъзМзДПіЯТЃЌ1.28gИУбѕЛЏЮяЕФЬхЛ§ЮЊ448mLЃЌИУЦјЬхЕФЮяжЪЕФСПЮЊЃК![]() =0.02molЃЌИУЦјЬхЕФФІЖћжЪСПЮЊЃК
=0.02molЃЌИУЦјЬхЕФФІЖћжЪСПЮЊЃК![]() =64g/molЃЌЩшRЕФЯрЖддзгжЪСПЮЊxЃЌдђx+16ЁС2=64ЃЌНтЖСx=32ЃЌRЮЊOдЊЫиЃЌЙЪД№АИЮЊЃК64g/molЃЛ32ЃЛ
=64g/molЃЌЩшRЕФЯрЖддзгжЪСПЮЊxЃЌдђx+16ЁС2=64ЃЌНтЖСx=32ЃЌRЮЊOдЊЫиЃЌЙЪД№АИЮЊЃК64g/molЃЛ32ЃЛ
ЃЈ5ЃЉ18gH2OЕФЮяжЪЕФСПЮЊ![]() =1molЃЌ1ИіH2OЗжзгжага3ИідзгЃЌЙЪКЌгадзгЪ§ФПЮЊ1molЁС3NAmol-1=3NAЃЛУПИіH2OЗжзгКЌга10ИіЕчзгЃЌЙЪnЃЈЕчзгЃЉ=1molЁС10=10molЃЌЙЪКЌгаЕчзгЪ§ФПЮЊ10molЁСNAmol-1=10NAЃЌЙЪД№АИЮЊЃК3NAЃЛ10NAЁЃ
=1molЃЌ1ИіH2OЗжзгжага3ИідзгЃЌЙЪКЌгадзгЪ§ФПЮЊ1molЁС3NAmol-1=3NAЃЛУПИіH2OЗжзгКЌга10ИіЕчзгЃЌЙЪnЃЈЕчзгЃЉ=1molЁС10=10molЃЌЙЪКЌгаЕчзгЪ§ФПЮЊ10molЁСNAmol-1=10NAЃЌЙЪД№АИЮЊЃК3NAЃЛ10NAЁЃ


 ПЊаФЭмзДдЊВтЪдОэЯЕСаД№АИ
ПЊаФЭмзДдЊВтЪдОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗжБ№ШМЩеЕШЮяжЪЕФСПЕФЯТСаИїзщЮяжЪЃЌЦфжаЯћКФбѕЦјЕФСПЯрЕШЕФЪЧЃЈ ЃЉ
ЂйC2H2гыC2H4O ЂкC4H8гыC6H12O6 ЂлC7H8гыC6H12 ЂмHCOOCH3гыCH3COOHЃЎ
AЃЎЂйЂлЂм BЃЎЂйЂкЂлЂм CЃЎЂйЂм DЃЎЂйЂкЂм
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗњЛЏИѕПЩгУзїУЋжЏЦЗЗРжћМСЁЂТБЛЏДпЛЏМСЁЂДѓРэЪЏгВЛЏМАзХЩЋМСЁЃвдИѕдЦФИПѓЪЏ(КЌ4.5%Cr2O3ЃЌЛЙКЌFe2O3ЁЂFeOЁЂMgOЁЂSiO2)ЮЊдСЯжЦБИЗњЛЏИѕЕФЙЄвеСїГЬШчЭМЁЃ
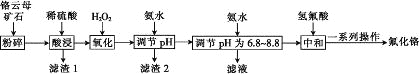
БэжаСаГіСЫЯрЙиН№ЪєРызгЩњГЩЧтбѕЛЏЮяГСЕэЕФpHЃК
ЧтбѕЛЏЮя | Fe(OH)3 | Mg(OH)2 | Cr(OH)3 | Fe(OH)2 |
ПЊЪМГСЕэЕФpH | 2.3 | 8.8 | 4.9 | 7.5 |
ГСЕэЭъШЋЕФpH | 4.1 | 10.4 | 6.8 | 9.7 |
ЧыЛиД№ЯТСаЮЪЬтЃК
(1)Fe2O3ЁЂMgOЁЂFeOЁЂSiO2жаЪєгкМюадбѕЛЏЮяЕФга________жжЁЃ
(2)НЋИѕдЦФИПѓЪЏЗлЫщЕФФПЕФЪЧ________ЁЃ
(3)ТЫдќ1жївЊГЩЗжЕФгУЭОЪЧ________ЁЃ(аДвЛжж)
(4)Cr2O3гыЯЁСђЫсЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________ЁЃ
(5)ЕквЛДЮЕЮМгАБЫЎЕїНкpHЗЖЮЇЮЊ________ЁЃ
(6)ЕкЖўДЮЕЮМгАБЫЎЕїНкpHЮЊ6.8~8.8ЕФФПЕФЪЧ_______ЃЌCr(OH)3гы Al(OH)3вЛбљОпгаСНадЃЌШєЕкЖўДЮЕЮМгЕФАБЫЎИФЮЊNaOHШмвКЃЌЩњГЩЕФCr(OH)3ЛсВПЗжШмНтЃЌаДГіCr(OH)3ШмНтЕФРызгЗНГЬЪНЃК________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУШчЭМЫљЪОзАжУЬНОПCl2КЭNO2дкNaOHШмвКжаЕФЗДгІЃЌШєЭЈШыЪЪЕББШР§ЕФCl2КЭNO2ЃЌМДЗЂЩњЗДгІCl2+2NO2+4NaOH=2NaNO3+2NaCl+2H2OЁЃ
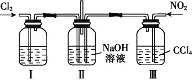
ЯТСаа№Ъіе§ШЗЕФЪЧ
A. ЪЕбщЪвжагУЖўбѕЛЏУЬгы3 molЁЄL-1ЕФбЮЫсЙВШШжЦБИТШЦј
B. зАжУЂёжаЪЂЗХЕФЪдМСЪЧХЈСђЫсЃЌзїгУЪЧИЩдяТШЦј
C. зАжУЂѓЕФзїгУЪЧБугкПижЦЭЈШыNO2ЕФСП
D. ШєжЦБИЕФNO2жаКЌгаNOЃЌгІНЋЛьКЯЦјЬхЭЈШыЫЎжавдГ§ШЅNO
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПДЮСзЫс(H3PO2)ЪЧвЛжжОЋЯИСзЛЏЙЄВњЦЗЃЌгаЧПЛЙдадЁЃвбжЊЃКЂй2P4+3Ba(OH)2+6H2O=3Ba(H2PO2)2+2PH3ЁќЃЌЂкH3PO2+NaOH(зуСП)=NaH2PO2+H2OЁЃЯТСаЭЦЖЯВЛе§ШЗЕФЪЧ
A. H3PO2ЕФНсЙЙЪНЮЊ
B. H3PO2ОпгаЧПЛЙдадЃЌдкПеЦјжаПЩФмБЛбѕЛЏГЩСзЫс
C. NaH2PO2ЪЧЫсЪНбЮ
D. УПЯћКФ1mol P4ЃЌЗДгІЂйжазЊвЦ6molЕчзг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГШмвКXКЌгаH+ЁЂAl3+ЁЂNH4+ЁЂFe2+ЁЂFe3+ЁЂHCO3-ЁЂS2-ЁЂSO42-ЁЂCl-ЁЂOH-жаЕФвЛжжЛђМИжжЃЌШЁИУШмвКНјааЪЕбщЃЌЪЕбщФкШнКЭЯрЙиЪ§Он(ЦјЬхЬхЛ§дкБъзМзДПіЯТВтЖЈ)ШчЯТЃК
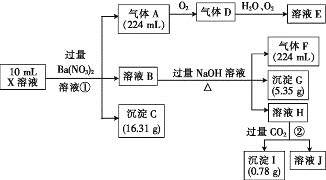
(1)ЭЈЙ§ЩЯЪіЪЕбщЙ§ГЬЃЌвЛЖЈВЛДцдкЕФРызгЪЧ_______ЁЃ
(2)ЗДгІЂйжаЩњГЩAЕФРызгЗНГЬЪНЮЊ________ЁЃ
(3)ШєВтЕУXШмвКжаc(H+)=6molЁЄL-1ЃЌдђXШмвКжа_______(ЬюЁАКЌЁБЛђЁАВЛКЌЁБ)Fe3+ЃЌc(Fe3+)=________molЁЄL-1(ШєЬюВЛКЌЃЌдђВЛашМЦЫу)ЃЌXШмвКжаc(Cl-)=_______molЁЄL-1ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖўМзУб(CH3OCH3)дкЮДРДПЩФмЬцДњВёгЭКЭвКЛЏЦјзїЮЊНрОЛвКЬхШМСЯЪЙгУЃЎ
ЙЄвЕЩЯвдCOКЭH2ЮЊдСЯЩњВњCH3OCH3ЕФаТЙЄвежївЊЗЂЩњШ§ИіЗДгІЃК
ЂйCO( g)+2H2(g)![]() CH3OH( g)ЁїH1=-91kJЁЄmol-1
CH3OH( g)ЁїH1=-91kJЁЄmol-1
Ђк2CH3OH(g)![]() CH3OCH3(g)+H2O(g)ЁїH2=-24kJЁЄmol-1
CH3OCH3(g)+H2O(g)ЁїH2=-24kJЁЄmol-1
ЂлCO(g)+H2O(g)![]() CO2(g)+H2(g)ЁїH3=-41kJЁЄmol-1
CO2(g)+H2(g)ЁїH3=-41kJЁЄmol-1
ЛиД№ЯТСаЮЪЬтЃК
(1)аТЙЄвеЕФзмЗДгІЮЊЃК3CO(g)+3H2(g)![]() CH3OCH3(g)+CO2(g)ЃЌИУЗДгІЁїH=______ЃЌвЛЖЈЬѕМўЯТЕФУмБеШнЦїжаЃЌИУзмЗДгІДяЕНЦНКтЃЌвЊЬсИпCOЕФзЊЛЏТЪЃЌПЩвдВЩШЁЕФДыЪЉЪЧ________(ЬюзжФИДњКХ)ЁЃ
CH3OCH3(g)+CO2(g)ЃЌИУЗДгІЁїH=______ЃЌвЛЖЈЬѕМўЯТЕФУмБеШнЦїжаЃЌИУзмЗДгІДяЕНЦНКтЃЌвЊЬсИпCOЕФзЊЛЏТЪЃЌПЩвдВЩШЁЕФДыЪЉЪЧ________(ЬюзжФИДњКХ)ЁЃ
aЃЎИпЮТИпбЙЁЁbЃЎМгШыДпЛЏМС cЃЎМѕЩйCO2ЕФХЈЖШdЃЎдіМгCOЕФХЈЖШЁЁeЃЎЗжРыГіЖўМзУб
(2)вбжЊЗДгІЂм2CH3OH(g)![]()
![]() CH3OCH3(g)ЃЋH2O(g)ЃЌФГЮТЖШЯТЃЌдк1LУмБеШнЦїжаМгШыCH3OHЃЌЗДгІ10minЪБДяЕНЦНКтЃЌДЫЪБИїзщЗжЕФХЈЖШШчЯТЃК
CH3OCH3(g)ЃЋH2O(g)ЃЌФГЮТЖШЯТЃЌдк1LУмБеШнЦїжаМгШыCH3OHЃЌЗДгІ10minЪБДяЕНЦНКтЃЌДЫЪБИїзщЗжЕФХЈЖШШчЯТЃК
ЮяжЪ | CH3OH | CH3OCH3 | H2O |
ХЈЖШ/(molЁЄLЃ1) | 0.01 | 0.2 | 0.2 |
ЂйЦНКтГЃЪ§БэДяЪНK=______ЃЌИУЮТЖШЕФЦНКтГЃЪ§ЮЊ______ЁЃ
ЂкШєМгШыCH3OHКѓЃЌО10 minКѓДяЕНЦНКтЃЌИУЪБМфФкЕФЦНОљЗДгІЫйТЪv(CH3OH)ЃН________ЁЃ
ЙЄвЕЩЯГЃНЋКЌЩщЗЯдќ(жївЊГЩЗжЮЊAs2S3)жЦГЩНЌзДЃЌЭЈШыO2бѕЛЏЃЌЩњГЩH3AsO4КЭЕЅжЪСђЁЃаДГіЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪН________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП25ЁцЪБ,Яђ25mL 0.1molЁЄL-1 NaOHШмвКжаж№ЕЮМгШы0.2 molЁЄL-1ДзЫсШмвК,ЕЮЖЈЧњЯпШчЭМЫљЪО,ЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
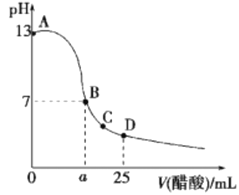
A. дкAЁЂBМфШЮвЛЕуЕФШмвКжавЛЖЈЖМга:c(Na+)>c(CH3COO-)>c(OH-)>c(H+)
B. ![]()
C. CЕуЖдгІЕФШмвКжа,ЫЎЕчРыГіЕФH+ХЈЖШДѓгк10-7molЁЄL-1
D. DЕуЖдгІЕФШмвКжа,ДцдкШчЯТЙиЯЕ:c(CH3COO-)-c(CH3COOH) =2c(H+) -c(OH-)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЙигкгаЛњЮяУќУћВЛе§ШЗЕФЪЧ
A. ![]() ЖдЖўМзБН
ЖдЖўМзБН
B. 2,3-ЖўМзЛљ-3-ввЛљЮьЭщ
C. ![]() вьЖЁЯЉ
вьЖЁЯЉ
D. (CH3)3CCH2CH(C2H5)CH3 2ЃЌ2-ЖўМзЛљ-4-ввЛљЮьЭщ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com