
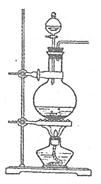
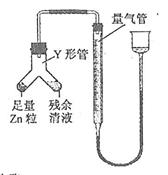
 Mn2��+Cl2��+2H2O�����Կ�����Ӧ����Һ��c(Cl��)>c(H��)���ü�����õ���c(Cl��)��������(H��)��
Mn2��+Cl2��+2H2O�����Կ�����Ӧ����Һ��c(Cl��)>c(H��)���ü�����õ���c(Cl��)��������(H��)��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
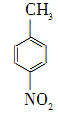 ��Na2Cr2O7��4H2SO4�D��
��Na2Cr2O7��4H2SO4�D�� ��Na2SO4��Cr2(SO4)3��5H2O
��Na2SO4��Cr2(SO4)3��5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
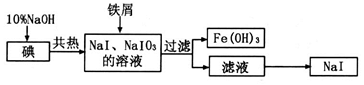
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
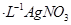 ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬����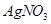 ��Һ�����ƽ��ֵΪ19��00mL��
��Һ�����ƽ��ֵΪ19��00mL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
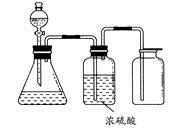
| A��ͭм��ϡ���� |
| B���������̺�Ũ���� |
| C����Ũ��ˮ����ʯ�ҷ�Ӧ |
| D��̼��ƺ�ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
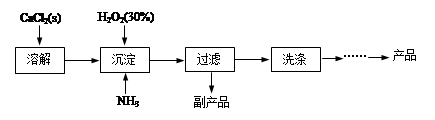

| �ζ����� | ��Ʒ������/g | KMnO4��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.3000 | 1.02 | 24.04 |
| 2 | 0.3000 | 2.00 | 25.03 |
| 3 | 0.3000 | 0.20 | 23.24 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cl2 | B��SO2 | C��H2 | D��NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����ͼ�У���ʹMnO2����������Ҳ����ȫ������ |
| B����ͼ�У�ʪ�����ɫ��������ɫ����������Һ�����ձ��У�����Һ�����ԣ������Cl2���� |
| C����ͼ�У�������ɫ���� |
| D����ͼ��:ˮ���Գ����Թ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com