

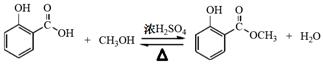
 (2��)��
(2��)��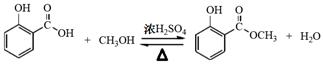 (2��)
(2��)


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
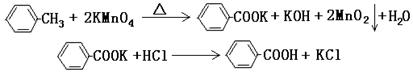
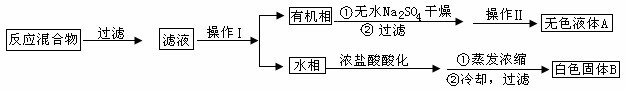
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ�У����ȣ��ܽ⣬�� | �õ���ɫ�������ɫ��Һ | �������� |
| �� | ȡ������Һ���Թ��У� �� | ���ɰ�ɫ���� | ��Һ����Cl- |
| �� | �����ɫ���壬 �� | �� | ��ɫ�����DZ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

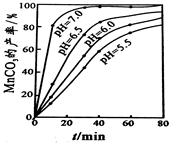
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

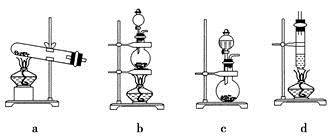
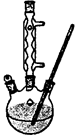
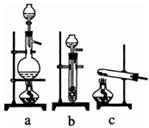
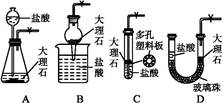
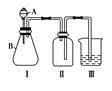 (4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺
(4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������������� | B�������¶� | C�����Ͻ��� | D�����̽���ʱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
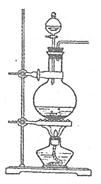
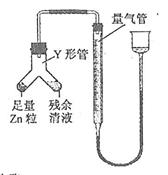
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
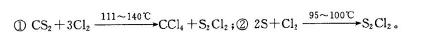 ��֪��S2Cl2����Ԫ����+1�ۣ�����ʽ��
��֪��S2Cl2����Ԫ����+1�ۣ�����ʽ��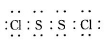 �������ȶ�����ˮ�������绯��Ӧ (һ������Ԫ�ؼ�̬���ߣ�һ���ֽ���)����Ӧ�漰�ļ������ʵ��۷е����£�
�������ȶ�����ˮ�������绯��Ӧ (һ������Ԫ�ؼ�̬���ߣ�һ���ֽ���)����Ӧ�漰�ļ������ʵ��۷е����£�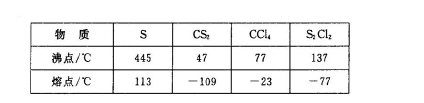
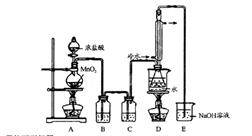
| A��ʯ�ͷ��� | B���Ʊ���ϩ | C����ȡ�������� | D����ȡ�屽 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com