
����Ŀ��A��B��C��D��E��F�Ǻ˵����������������ֶ���������Ԫ�أ�AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ�����4��C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��C��Eͬ���塣
(1)B�����ڱ��е�λ��______________________________________________
(2)FԪ�ص�����������Ӧ��ˮ����Ļ�ѧʽΪ___________________________________��
(3)Ԫ��C��D��E�γɵļ����Ӱ뾶��С�����˳��________________________(�����ӷ��ű�ʾ)��
(4)�õ���ʽ��ʾ������D2C���γɹ��̣�__________________________________________________��
C��D�����γɻ�����D2C2��D2C2�к��еĻ�ѧ����_________________________________________��
(5)C��E���⻯��е��ɸߵ���˳���ǣ�_______________________________��
(6)д��̼������E������������Ӧˮ����Ũ��Һ��Ӧ�Ļ�ѧ����ʽ�����õ����ű������ӵ�ת�Ʒ���_______________����ת�Ƶ���Ϊ0.2molʱ����״���·�Ӧ��������_______________L��
(7)��֪E���ʺ�F���ʵ�ˮ��Һ��Ӧ����������ǿ�ᣬ�����ӷ���ʽΪ_________________��
���𰸡��ڶ����ڵ���A�� HClO4 r(Na+) ��r(O2��)�� r(S2��)��Na+ ��O2����S2�� ![]() ���Ӽ������ۼ�(��Ǽ��Լ�) H2O��H2S
���Ӽ������ۼ�(��Ǽ��Լ�) H2O��H2S 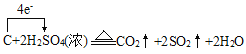 3.36 S��3Cl2��4H2O = 8H+��6Cl����SO42-
3.36 S��3Cl2��4H2O = 8H+��6Cl����SO42-
��������
AԪ�ص�ԭ�Ӻ���ֻ��һ�����ӣ��ɴ˿�֪AΪH������ B������������Ӧˮ����Ļ�ѧʽΪHBO3 ����֪B�����Ϊ+5�ۣ���VA���ٸ���BԪ�ص�ԭ�Ӱ뾶����������������С�ģ��ó�BΪN������CԪ��ԭ�ӵ������������ȴ�����������4���ó�CΪO��C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C����֪D��������Ϊ+1�������ӣ�����DΪNa��C��Eͬ���壬��EΪ������Ԫ�أ��ó�EΪS��F�Ƕ�����Ԫ������SԪ�غ��������Ԫ�أ�Fֻ��ΪCl�����Ͽ�֪AΪH��BΪN��CΪO��DΪNa��EΪS��FΪCl���ݴ˻ش�
��1��BΪ��Ԫ�أ�Nλ�����ڱ��ڶ����ڵ�VA�壬�ʴ�Ϊ���ڶ����ڣ�VA��
��2��FΪClԪ�أ�ClԪ������������ˮ������HClO4���ʴ�Ϊ��HClO4��
��3��Ԫ��C��D��E�γɵļ����ӷֱ�ΪO2����Na+��S2�������Ӳ���������Ӱ뾶�ϴ�������Ų���ͬ�����������Ӱ뾶��ԭ���������������С�����뾶��С����Ϊr(Na+) ��r(O2��)�� r(S2��)���ʴ�Ϊ��r(Na+) ��r(O2��)�� r(S2��)��
��4��D2C��Na2O���õ���ʽ��ʾ�γɹ���Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��D2C2ΪNa2O2�����к��еĻ�ѧ��Ϊ���Ӽ������ۼ����ʴ�Ϊ�����Ӽ������ۼ���
��D2C2ΪNa2O2�����к��еĻ�ѧ��Ϊ���Ӽ������ۼ����ʴ�Ϊ�����Ӽ������ۼ���
��5��C��E���⻯��ֱ�ΪH2O��H2S������H2O����֮�������������·е�Ҫ���ߣ����Էе�H2O��H2S���ʴ�Ϊ��H2O��H2S��
��6��C��Ũ���ᷴӦ����ʽΪ��C+2H2SO4(Ũ) ![]() 2SO2��+CO2��+2H2O�������������������ת��
2SO2��+CO2��+2H2O�������������������ת��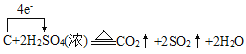 ����ת��4mole-ʱ��������SO2 2mol��CO2 1mol����3mol����״�������Ϊ67.2L����ת��0.2mol����ʱ���������Ϊ
����ת��4mole-ʱ��������SO2 2mol��CO2 1mol����3mol����״�������Ϊ67.2L����ת��0.2mol����ʱ���������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��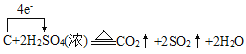 ��3.36L��
��3.36L��
��7��S����ˮ��Ӧ�Ļ�ѧ����ʽΪ3Cl2+S+ 4H2O=6HCl+H2SO4���������ӷ���ʽΪS��3Cl2��4H2O = 8H+��6Cl����SO42-���ʴ�Ϊ��S��/span>3Cl2��4H2O = 8H+��6Cl����SO42-��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A.F2��Cl2��Br2��I2 �۵���ԭ�������ĵ���������
B.���ڷǽ�����Cl>Br>I����������HCl>HBr>HI
C.ԭ������Ϊ24��Ԫ�����ڳ����ڵĸ���Ԫ��
D.����Cs�ڿ�����ȼ�յIJ�����Cs2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���25.00mL 0.1mol��L��1 HSCN��Һ�е���0.1 mol��L��1 NaOH��Һ����Һ����ˮ�������c(H+)�ĸ�����[��1gcˮ(H+)]������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��

A. �����£�Ka(HSCN)ԼΪ1��10��3
B. N����Һ�У�c(Na+)��c(SCN��)
C. R��Q������Һ��Ӧ��[H+]��Ϊ10��7
D. b��25.00
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ù�ҵ�ϼ���(��Ҫ�ɷ�Na2CO3)�������᳧β���е�SO2�Ʊ���ˮNa2SO3���������£�

����˵���������( )
A. ��������ŷ�SO2���ܵ������귢��
B. �к����з�����Ӧ�����ӷ���ʽΪHSO3-��OH��= SO32-��H2O
C. ����Na2SO3��Ʒ���Ƿ�Na2SO4����ѡ��ϡ�����Ba(NO3)2��Һ
D. �������Ļ��ķ�ɢϵ������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ҫ����A12O3��Fe2O3��FeO��SiO2���������Ʊ���ˮ������KAl(SO4)2��12H2O�Ͳ�Ѫ��FeSO4��7H2O��������������(���ֲ����Ͳ�����ȥ)��
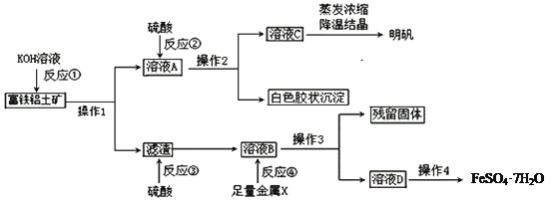
(1)����1����Ҫ�õ��IJ�������______________________��
(2)��Ӧ�٢ڢۢ�����������ԭ��Ӧ����_________����д��ţ���
(3)�ۺϿ��ǣ�����X���ѡ��__________��д���÷�Ӧ���ӷ�����ʽ_________________________��
(4)��Ӧ�ٵ����ӷ���ʽ��___________________��___________________________��
(5)��ҺD�к��еĽ�����������_______�����鷽����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�������ķ���ʽΪC10H14��������ʹ��ˮ��ɫ����ʹ����KMnO4��Һ��ɫ�����ӽṹ��ֻ����һ���������������������У� ��
A.2��B.3��C.4��D.5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б���������ǣ� ��
A. CS2�ĽṹʽΪ��S��C��S
B. 6CO2��6H2O![]() C6H12O6��6O2 ���ñ仯�й���ֱ��ת��Ϊ��ѧ��
C6H12O6��6O2 ���ñ仯�й���ֱ��ת��Ϊ��ѧ��
C. CO2��g����C��s��![]() 2CO��g����H��0����S��0���÷�Ӧ���������Է�����
2CO��g����H��0����S��0���÷�Ӧ���������Է�����
D. NH3ˮ��Һ�ʼ��Ե�ԭ����NH3��H2O![]() NH3��H2O
NH3��H2O![]() NH4����OH��
NH4����OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���縡ѡ���۷��ǹ�ҵ�ϲ��õ�һ����ˮ������������������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe(OH)3���壬Fe(OH)3��������������ã�������ˮ�е������ʹ�������������ˮ�����ã���ԭ����ͼ��ʾ������˵����ȷ����

A. ʯī�缫�Ϸ���������Ӧ
B. ����ͼʾ������AΪCO2
C. Ϊ��ǿ��ˮ�ĵ���������������ˮ�м��������Ҵ�
D. ����ȼ�ϵ����CO32�������һ���ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100 g 5.00%��NaOH��Һ��������CuSO4��Һ��100 g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��

��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ��ݴ˻ش����⣺
�ٵ�Դ��N��Ϊ�ߣߣߣߣߣ���
�ڵ缫b�Ϸ����ĵ缫��ӦΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����ʽ����缫b�����ɵ������ڱ�״���µ������
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ܵ缫c�������仯�ǣߣߣߣߣߣߣߣߣ�g��
�ݵ��ǰ�����Һ���ᡢ���Դ�С�Ƿ����仯��������ԭ��
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com