
 2O(l)====H2O(g)����H=+44kJ��mol-1����16 gҺ̬��ȼ�����ɵ�����Һ̬ˮʱ���ų���������________kJ��
2O(l)====H2O(g)����H=+44kJ��mol-1����16 gҺ̬��ȼ�����ɵ�����Һ̬ˮʱ���ų���������________kJ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ں���ת��Ϊ�����Ƿ��ȷ�Ӧ���������ĺ��������Ȱ��� |
| B��ϡ������ϡNaOH��Һ��Ӧ���к��ȡ�H��57.3 kJ/mol |
| C��̼��ȼ���ȴ���-110.5 kJ/mol |
| D��ϡ������ϡNaOH��Һ��Ӧ����1 molˮ����H ���� ��57.3 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2SO3��g������H =" a" kJ/mol����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g����ȫת��Ϊ1mol SO3��g������99kJ����ش�
2SO3��g������H =" a" kJ/mol����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g����ȫת��Ϊ1mol SO3��g������99kJ����ش�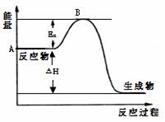
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
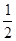 O2(g)=H2O(l) ��H3="-285.8KJ/mol "
O2(g)=H2O(l) ��H3="-285.8KJ/mol " | A��-488.3 KJ/mol | B��+488.3KJ/mol |
| C��-245.7KJ/mol | D��+245.7KJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����10 NA������ת��ʱ���÷�Ӧ�ų�1300kJ������ |
| B����1 NA��ˮ����������ΪҺ��ʱ������1300kJ������ |
| C����2 NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
| D����8 NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH (g) ��H 1����Ӧ��
CH3OH (g) ��H 1����Ӧ��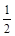 O2(g)��CO2(g) ��H 2����283 kJ��mol��
O2(g)��CO2(g) ��H 2����283 kJ��mol�� 1 (��Ӧ��)
1 (��Ӧ��)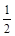 O2(g)��H2O(g) ��H3����242 kJ��mol��1 (��Ӧ��)
O2(g)��H2O(g) ��H3����242 kJ��mol��1 (��Ӧ��)| ��ѧ�� | C��C | C��H | H��H | C��O | C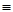 O O | H��O |
| ���ܣ�kJ��mol��1 | 348 | 413 | 436 | 358 | 1072 | 463 |
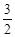 O2(g)��CO2(g)��2H2O(g) ��H4
O2(g)��CO2(g)��2H2O(g) ��H4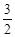 O2(g)��CO2(g)��2H2O(l)���乤��ԭ��ʾ��ͼ���£�
O2(g)��CO2(g)��2H2O(l)���乤��ԭ��ʾ��ͼ���£�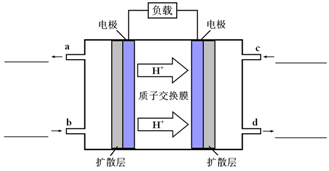
 ѧʽ��
ѧʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2N2H4(g)��2NO2(g)===3N2(g)��4H2O(l) ��H����1135.7 kJ��mol��1 |
| B��2N2H4(g)��2NO2(g)===3N2(g)��4H2O(g) ��H����1000.3 kJ��mol��1 |
| C��N2H4(g)��NO2(g)===3/2N2(g)��2H2O(l) ��H����1135.7 kJ��mol��1 |
| D��2N2H4(g)��2NO2(g)===3N2(g)��4H2O(g) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�����ѽ��γ�ȼ�� | B��¶����� |
| C����Ϊ�л����Ͻ������ϵ�ԭ�� | D��ֱ������ |
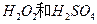 �Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��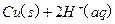 ====
====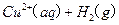
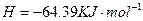
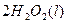 ====
====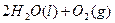
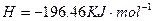
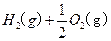 ="==="
="===" 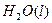
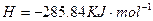
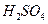 ��Һ��
��Һ��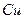 ��
��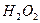 ��Ӧ����
��Ӧ����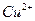 ��
��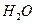 ���Ȼ�����ʽΪ ��
���Ȼ�����ʽΪ ��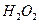 ��3.0
��3.0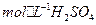 �Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
�Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭƽ���ܽ����� �� 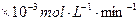 ��[:.....] ��[:.....] | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
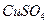 ��Һ�м���һ������
��Һ�м���һ������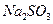 ��
��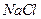 ��Һ�����ȣ�����
��Һ�����ȣ�����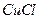 �������Ʊ�
�������Ʊ�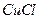 �����ӷ���ʽ�� ��
�����ӷ���ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
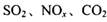 �ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;����
�ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;���� Ũ�ȵ���__________ (ѡ����ĸ)��
Ũ�ȵ���__________ (ѡ����ĸ)��


 �����£�
�����£�
 ,��ƽ�ⳣ��K=13.3��
,��ƽ�ⳣ��K=13.3�� ����
���� =_______________(������λ��Ч����)��
=_______________(������λ��Ч����)��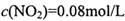 ��
�� ����ı��������____________________
����ı��������____________________�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com