
���ⶨ�����е�SO2��������������ʵ�飺ȡ100 L�ÿ���(��״��)��ͨ��ʢ��100 mL��������(H2O2)ˮ��Һ������ƿ��ʹ�����е�SO2��ȫ���գ��������ᡣ�ڷ�Ӧ���ˮ��Һ�м�������BaCl2��Һ�����ɰ�ɫ���������ⶨ������Ϊ11.65g�������������ɷֶԲⶨ�����Ӱ�죩��
(1)д��SO2��H2O2��Ӧ�����ӷ���ʽ��
(2)д��H2SO4��BaCl2��Ӧ�����ӷ���ʽ��
(3)��100 L������SO2�����������
��1��SO2+H2O2��2H++SO42-��2�֣���2��SO42-+Ba2+��BaSO4����2�֣���3��1.12%��2�֣�
���������������1��SO2���л�ԭ�ԣ�˫��ˮ���������ԣ����߷���������ԭ��Ӧ����Ӧ�����ӷ���ʽ��SO2+H2O2��2H++SO42-��
��2��ϡ������Ȼ�����Ӧ�������ᱵ��ɫ��������Ӧ�����ӷ���ʽ��SO42-+Ba2+��BaSO4����
��3�����ᱵ��������11.65g�����ʵ�����11.65g��233g/mol��0.05mol
��˸���Sԭ���غ��֪��SO2�����ʵ���Ҳ��0.05mol
��100 L������SO2�����������
���㣺����SO2�����ʡ�������SO2�����IJⶨ
�����������ǻ���������Ŀ��飬�ѶȲ����ض�ѧ������֪ʶ�Ĺ��̣�����������ѧ������˼ά�����淶��������������Ĺؼ������ú�ԭ���غ㣬�غ㷨�ǻ�ѧ��������õķ�������Ҫ��ƽʱ��ѧϰ��ע����ۺ��ܽᡢ���ɡ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��������͵������NOx���Ǵ�������Ҫ��Ⱦ�����������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��
������м��㣺
��1��ʯ��ʯ-ʯ��ʪ�����������ռ�����ԭ���ǣ������еĶ��������뽬Һ�е�̼����Լ�������Ӧ����ʯ�ࣨCaSO4��2H2O����ij�糧��ú300t��ú�к������������Ϊ2.5%������ȼ��ʱú�е���ȫ��ת��Ϊ���������ø÷�������ʱ��96%����ת��Ϊʯ�࣬�������Ͽɵõ�_________��ʯ�ࡣ
��2����CH4����ԭNOx����Ҫ����ΪN2��CO2������1 L NOx����NO2��NO������CH4���仹ԭ��N2������ͬ��ͬѹ�µ�CH4 0.4 L������������NO2��NO��������֮��Ϊ____��
��3���Ҷ��ᣨH2C2O4�����Ʊ������·�Ӧ��
C6H12O6��18HNO3��3H2C2O4��18NO2����12H2O
C6H12O6��6HNO3��3H2C2O4��6NO����6H2O
������Ӧ������NOx��������ˮ���պ���������ѭ�����ã���β��NOx��n(NO2)�Un(NO)��2�U1����NOx������ת����Ϊ90%��ÿ����9 kg�Ҷ���������Ҫ������������Ϊ63%��������Һ����ǧ�ˣ�
��4���ü�Һ���շ�����ij��ҵβ������NOx��NO��NO2��N2O4���ķ�ӦΪ��
2NO2 + 2NaOH�� NaNO2 + NaNO3 + H2O��
NO + NO2 + 2NaOH�� 2NaNO2 + H2O��
��N2O4�������Ϊ0.2ʱ������1mol��NOx����������NaNO3��NaNO2�����ʵ�����x����Ϊ��֪����ʹ�ã���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ȡһ������Fe��Cu�Ļ�����ĩ��ƽ���ֳ���ȷݣ��ֱ���ÿһ���м���һ������ϡ���ᣬ���跴Ӧ�Ļ�ԭ����ֻ��NO����ʵ�������ɵ�NO�������������ʣ������������¼����(����������ڱ�״���²ⶨ)��
| ʵ����� | 1 | 2 | 3 | 4 | 5 |
| ������Һ��� | 100 mL | 200 mL | 300 mL | 400 mL | 500 mL |
| ʣ���������� | 17.2 g | 8 .0g | 0 g | 0 g | 0 g |
| ������� | 2.24 L | 4.48 L | 6.72 L | 7.84 L | 7.84 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һ����������Ļ����Һ��ȡ��10mL��������BaCl2��Һ�����ˡ�ϴ�ӡ���ɺ�õ�9.32g�ij�������Һ��4.0mol��L-1NaOH��Һ��Ӧ����ȥ35mL��Һʱǡ����ȫ�к͡������Һ��H2SO4��HNO3�����ʵ���Ũ�ȸ��Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��1��NAΪ�����ӵ�������25��ʱ��1gˮ�к�H+���Ӹ���ԼΪ__________NA��
��2��ͨ�����Ĵ���������ȡ���ᣬ�ڴ�ȫ�����У������ϰ����������������ʵ�����Ϊ__________,���������������������Ϊ_____________��
��3������0��2 mol NaOH��0��1 mol Ca(OH)2�Ļ����Һ�г����ȶ���ͨ��CO2����0��5 mol������CO2����Ϊ�����꣬����Һ�����ӵ�����Ϊ�����꣬��������������CO2�������仯������ͼ��(����������ʵĵ�����ε�ˮ��)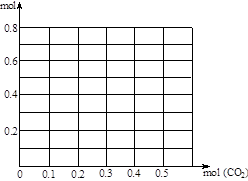
��4��ij�о���ѧϰС������ͭм������ͭ������������������ɵĻ��ᷴӦ����ȡCuSO4��5H2O���壬����������Ļ�ԭ����ΪNO����Ӧ�����в�����SO2����Ӧ�����Һ�в���Cu��NO3��2�� ��Ӧ�й�����ȫ�ܽ⣬�������ǡ����ȫ��Ӧ������������������Ϊ480 g������ͭм����������Ϊ0��4�� 480g����������һ���������Ⱥ�ַ�Ӧ����ȴǡ��ֻ�õ�CuSO4��5H2O������ԭ������H2SO4������������д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
þ�����������仯�������������������й㷺��Ӧ�á�
��1��þ���Ͻ����ڷɻ�����ҵ������3��90��þ���Ͻ�����������2mol/Lϡ����������0��2mol���������㲢ȷ��þ���Ͻ������ʵ���n(Mg): n(Al)= ��
��2�����������Ҫ�ɷ�ΪFeS2(��������ֻ��SiO2)�����������ԭ�ϡ�ȡij������10g�������Ŀ��������գ�4FeS2+11O2��2Fe2O3+8SO2������ַ�Ӧ����ȴ���Ƶù�������Ϊ7��4g������SiO2����Ӧ��������������FeS2����������Ϊ ��
��3������һ���������ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ����Ӧ���������κ�����ų�������Ӧ��ij�����Һ������4��00 mol��L��1��NaOH��Һ������NaOH��Һ���������������������Ĺ�ϵ��ͼ��ʾ(��Ҫʱ�ɼ��ȣ�����������ˮ�е��ܽ�)����������A�����ֵ�� ��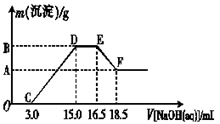
��4���������������������������֡�ȡij������ĩ28��12g(����ֻ��Fe��C)�����������г�ַ�Ӧ���õ�CO2����224mL(��״����)��
�ټ���˸�����ĩ������̼�����ʵ���֮��Ϊ ����������ȣ���
����ȡ���ݲ�ͬ����������������ĩ�ֱ�ӵ�100mL��ͬŨ�ȵ�ϡH2SO4�У���ַ�Ӧ��õ�ʵ���������±���ʾ��
| ʵ����� | �� | �� | �� |
| ���������ĩ��������g�� | 2��812 | 5��624 | 8��436 |
| ��������������L������״���� | 1��120 | 2��240 | 2��800 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС�����ⶨ������(25 �桢101 kPa)������Ħ���������ش��������⡣
��С����Ƶļ���ʵ��װ����ͼ��ʾ��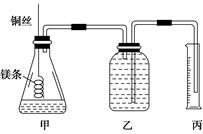
��ʵ�����Ҫ�����������£�
������100 mL 1.0 mol/L��������Һ��
����________(���������Ʋ�ע�����)��ȡ10.0 mL 1.0 mol/L��������Һ������ƿ�У�
�۳�ȡa g�ѳ�ȥ��������Ĥ��þ������ϵ��ͭ˿ĩ�ˣ�ΪʹHClȫ���μӷ�Ӧ��a����ֵ����Ϊ________��
�������ƿ��װ������ˮ������ͼ���Ӻ�װ�ã����װ�õ������ԣ�
�ݷ�Ӧ���������ϵ�¶Ȼָ������£�������Ͳ��ˮ�����ΪV mL��
�뽫�������貹���������ش��������⡣
(1)�����ֱ���ʵ�鲽����м��װ�������Եķ�����
___________________________________________________
(2)ʵ�鲽�����Ӧѡ��________(�����)����Ͳ��
A��100 mol���������� B��200 mL���������� C��500 mL
����ʱ���ָ��������⣬��Ҫע��_________________________________________��
(3)������ˮ������Ӱ�죬��ʵ�������²������Ħ������ļ���ʽΪVm��________����δ��ȥþ�����������Ĥ����������________(�ƫ����ƫС������Ӱ�족)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ��������CuSO4��5H2O������480mL0.1mol/L��CuSO4��Һ����ش��������⣺
��1��Ӧ��������ƽ��ȡCuSO4��5H2O����_______g��
��2�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ�1g����ʹ�����룩����ƽƽ��ʱ��ʵ�ʳ�����CuSO4��5H2O������__________g��
��3����ʵ���õ�����Ҫ�����У�������ƽ����Ͳ���ձ�����������________��________��
��4�����ƹ������м����ؼ��IJ���Ͳ�������ͼ��ʾ������Щʵ�鲽��A��F��ʵ������Ⱥ������
�� ��
���ж��ݵľ�������� ��
��5�����������ʹ������Һ��Ũ�Ȳ�������Ӱ�죨A.ƫ�� B.ƫ�� C.����,����š�����
���ܽ⾧���õ��ձ��Ͳ�����δϴ�ӣ�____________��
�ڶ���ʱ���ӿ̶��ߣ�____________��
������CuSO4��5H2O������ʧȥ���ֽᾧˮ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ڶ�����»ᡰ�Ҹ�֮�š���������Ͻ�������졣���Ͻ�����
| A���� | B�������� | C�������� | D������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com