
ĄūĖâÄŋĄŋ°īŌŠĮóÔÚÏÂÁÐŋÕļņÖÐĖîŋÕĄĢ
ĢĻ1ĢĐŌŧķĻÁŋĩÄŌŌīžÓëą―ĩÄŧėšÏÎïÓëŨãÁŋĩÄ―ðĘôÄÆ·īÓĶĢŽŋÉÉúģÉ11.2LĮâÆøĢĻÔÚąęŨžŨīŋöÏÂĢĐĢŽ―ŦīËŧėšÏÎïČžÉÕÄÜÉúģÉ108gËŪĄĢ
ĒŲĮóŧėšÏÎïÖÐą―ĩÄÖĘÁŋ__________________
ĢĻ2ĢĐÄģÖÖĖþAĩÄÕôÆøÕÛšÏģÉąęŋöÏÂĩÄÃÜķČĘĮ3.214g/LĢŽŌŅÖŠļÃĖþĩÄĖžĮâÖĘÁŋąČΊ5ĄÃ1ĢŽĮóĢš
ĒŲļÃĖþĩÄÏāķÔ·ÖŨÓÖĘÁŋĢš_________,
ĒÚČįđûļÃĖþĩÄŌŧÂČČĄīúÎïÓÐ4ÖÖĢŽÐīģöļÃĖþĩÄ―áđđžōĘ―Ģš___________________________________________
ĢĻ3ĢĐ5.8gÓÐŧúÎïÍęČŦČžÉÕĢŽÖŧÉúģÉCO2šÍH2OÕôÆøĢŽÆäĖåŧýąČΊ1:1(ÍŽŅđÍŽÎÂ)ĢŽČô°ŅËüÃĮÍĻđýžîĘŊŧŌĢŽžîĘŊŧŌÖĘÁŋÔöžÓ18.6gĢŽÍŽÁŋĩÄÓÐŧúÎïÓë0.1molŌŌËáÍęČŦ·ĒÉúõĨŧŊ·īÓĶĢŪÓÖÖŠļÃÓÐŧúÎïķÔŋÕÆøĩÄÏāķÔÃÜķČΊ2ĄĢĢĻŨĒŌâĢšôĮŧųēŧÄÜÖą―ÓÁŽÔÚËŦžüÉÏĢĐ
ĒŲÓÐŧúÎïĩÄ·ÖŨÓĘ―___________________________________________________________
ĒÚÓÐŧúÎïĩÄ―áđđžōĘ―___________________________________________________________
Ąūīð°ļĄŋ78g 72 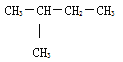 C3H6O CH2=CHCH2OH
C3H6O CH2=CHCH2OH
Ąū―âÎöĄŋ
ĢĻ1ĢĐąęŋöÏÂ11.2LĮâÆøĩÄÎïÖĘĩÄÁŋΊĢš![]() =0.5molĢŽÔōŧėšÏÎïÖКŽÓÐC2H5OHĩÄÎïÖĘĩÄÁŋΊĢš0.5molĄÁ2=1molĢŧ1molŌŌīžÍęČŦČžÉÕŧáÉúģÉËŪĩÄÎïÖĘĩÄÁŋΊĢš
=0.5molĢŽÔōŧėšÏÎïÖКŽÓÐC2H5OHĩÄÎïÖĘĩÄÁŋΊĢš0.5molĄÁ2=1molĢŧ1molŌŌīžÍęČŦČžÉÕŧáÉúģÉËŪĩÄÎïÖĘĩÄÁŋΊĢš![]() =3molĢŽÖĘÁŋΊĢš18g/molĄÁ3mol=54gĢŽŧėšÏÎïČžÉÕÄÜÉúģÉ108gËŪĢŽÔōŧėšÏÎïÖÐą―ÍęČŦČžÉÕÉúģÉËŪĩÄÖĘÁŋΊĢš108g-54g=54gĢŽËŪĩÄÎïÖĘĩÄÁŋΊĢš
=3molĢŽÖĘÁŋΊĢš18g/molĄÁ3mol=54gĢŽŧėšÏÎïČžÉÕÄÜÉúģÉ108gËŪĢŽÔōŧėšÏÎïÖÐą―ÍęČŦČžÉÕÉúģÉËŪĩÄÖĘÁŋΊĢš108g-54g=54gĢŽËŪĩÄÎïÖĘĩÄÁŋΊĢš![]() =3molĢŽ3molËŪÖКŽÓÐ6molHÔŨÓĢŽÔōą―ĩÄÎïÖĘĩÄÁŋΊĢš
=3molĢŽ3molËŪÖКŽÓÐ6molHÔŨÓĢŽÔōą―ĩÄÎïÖĘĩÄÁŋΊĢš![]() =1molĢŽËųŌÔĢŽŧėšÏÎïÖКŽÓÐC2H5OHĩÄÎïÖĘĩÄÁŋΊ1molĢŧŧėšÏÎïÖÐą―ĩÄÖĘÁŋΊĢš78g/molĄÁ1mol=78gĄĢđĘīð°ļΊĢš78gĄĢ
=1molĢŽËųŌÔĢŽŧėšÏÎïÖКŽÓÐC2H5OHĩÄÎïÖĘĩÄÁŋΊ1molĢŧŧėšÏÎïÖÐą―ĩÄÖĘÁŋΊĢš78g/molĄÁ1mol=78gĄĢđĘīð°ļΊĢš78gĄĢ
ĢĻ2ĢĐĒŲļÃĖþĩÄĖžĮâÖĘÁŋąČΊ5Ģš1ĢŽCĄĒHÔŠËØĩÄÎïÖĘĩÄÁŋÖŪąČΊĢš![]() Ģš
Ģš![]() =5Ģš12ĢŽļÃĖþĩÄŨîžōĘ―ÎŠĢšC5H12ĢŧļÃĖþĩÄÄĶķûÖĘÁŋΊĢšM=ĶŅVm=3.214g/LĄÁ22.4L/mol=72g/molĢŽžīļÃĖþĩÄÏāķÔ·ÖŨÓÖĘÁŋΊ72ĢŽđĘīð°ļΊĢš72ĄĢ
=5Ģš12ĢŽļÃĖþĩÄŨîžōĘ―ÎŠĢšC5H12ĢŧļÃĖþĩÄÄĶķûÖĘÁŋΊĢšM=ĶŅVm=3.214g/LĄÁ22.4L/mol=72g/molĢŽžīļÃĖþĩÄÏāķÔ·ÖŨÓÖĘÁŋΊ72ĢŽđĘīð°ļΊĢš72ĄĢ
ĒÚļÃĖþĩÄÏāķÔ·ÖŨÓÖĘÁŋΊ72ĢŽËųŌÔļÃÓÐŧúÎïĩÄ·ÖŨÓĘ―ÎŠĢšC5H12ĄĢC5H12ΊÎėÍéĢŽÎėÍéĩÄÍŽ·ÖŌėđđĖå·ÖΊÕýÎėÍéĄĒŌėÎėÍéšÍÐÂÎėÍéĢŽÆäÖÐÕýÎėÍéĩÄŌŧÂČīúÎïÓÐ3ÖÖĄĒŌėÎėÍéĩÄŌŧÂČīúÎïĩÄÍŽ·ÖŌėđđĖåÓÐ4ÖÖĄĒÐÂÎėÍéĩÄŌŧÂČīúÎïĩÄÍŽ·ÖŌėđđĖåÓÐ1ÖÖĢŽËųŌÔÂúŨãĖõžþĩÄĖþĩÄÍŽ·ÖŌėđđĖåΊĢš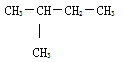 ĢŽđĘīð°ļΊĢš
ĢŽđĘīð°ļΊĢš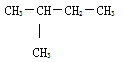 ĄĢ
ĄĢ
ĢĻ3ĢĐĒŲļÃÓÐŧúÎïķÔŋÕÆøĩÄÏāķÔÃÜķČΊ2ĢŽÔōÓÐŧúÎïÏāķÔ·ÖŨÓÖĘÁŋ=29ĄÁ2=58ĢŽ5.8gÓÐŧúÎïĩÄÎïÖĘĩÄÁŋΊ![]() =0.1molĢŽŌĀūÝČžÉÕÉúģÉCO2šÍH2OÕôÆøĮŌĖåŧýąČΊ1Ģš1ĢŽžîĘŊŧŌÖĘÁŋÔöžÓ18.6gΊCO2šÍH2OĩÄÖĘÁŋĢŽÔōn(CO2)=n(H2O)=
=0.1molĢŽŌĀūÝČžÉÕÉúģÉCO2šÍH2OÕôÆøĮŌĖåŧýąČΊ1Ģš1ĢŽžîĘŊŧŌÖĘÁŋÔöžÓ18.6gΊCO2šÍH2OĩÄÖĘÁŋĢŽÔōn(CO2)=n(H2O)=![]() =0.3molĢŽÔōÓÐŧúÎï·ÖŨÓÖÐN(C)=
=0.3molĢŽÔōÓÐŧúÎï·ÖŨÓÖÐN(C)=![]() =3ĄĒN(H)=
=3ĄĒN(H)=![]() =6ĄĒN(O)=
=6ĄĒN(O)=![]() =1ĢŽđĘÓÐŧúÎï·ÖŨÓĘ―ÎŠĢšC3H6OĢŽđĘīð°ļΊĢšC3H6OĄĢ
=1ĢŽđĘÓÐŧúÎï·ÖŨÓĘ―ÎŠĢšC3H6OĢŽđĘīð°ļΊĢšC3H6OĄĢ
ĒÚÍŽÁŋĩÄÓÐŧúÎïÓë0.1molŌŌËáÍęČŦ·ĒÉúõĨŧŊ·īÓĶĢŽÔōÓÐŧúÎï·ÖŨÓšŽÓÐ1ļö-OHĢŽÓÐŧúÎï·ÖŨÓĘ―ÎŠC3H6OĢŽšŽÓÐ1ļöC=CËŦžüĢŽđĘÆä―áđđžōĘ―ÎŠCH2=CHCH2OHĄĢđĘīð°ļΊĢšCH2=CHCH2OHĄĢ


 ÖąÍĻđóÖÝÃûÐĢÖÜēâÔÂŋžÖąÍĻÃûÐĢÏĩÁÐīð°ļ
ÖąÍĻđóÖÝÃûÐĢÖÜēâÔÂŋžÖąÍĻÃûÐĢÏĩÁÐīð°ļ
| Äęžķ | ļßÖÐŋÎģĖ | Äęžķ | ģõÖÐŋÎģĖ |
| ļßŌŧ | ļßŌŧÃâ·ŅŋÎģĖÍÆžöĢĄ | ģõŌŧ | ģõŌŧÃâ·ŅŋÎģĖÍÆžöĢĄ |
| ļßķþ | ļßķþÃâ·ŅŋÎģĖÍÆžöĢĄ | ģõķþ | ģõķþÃâ·ŅŋÎģĖÍÆžöĢĄ |
| ļßČý | ļßČýÃâ·ŅŋÎģĖÍÆžöĢĄ | ģõČý | ģõČýÃâ·ŅŋÎģĖÍÆžöĢĄ |
ŋÆÄŋĢšļßÖÐŧŊŅ§ ĀīÔīĢš ĖâÐÍĢš
ĄūĖâÄŋĄŋ·ÖŨÓĘ―ÎŠC3H7BrĩÄÓÐŧúÎïžŨÔÚĘĘŌËĩÄĖõžþÏÂÄÜ·ĒÉúČįÏÂŌŧÏĩÁÐŨŠŧŊĢš

(1)ČôBÄÜ·ĒÉúŌøūĩ·īÓĶĢŽĮëŧØīðÏÂÁÐÎĘĖâĢš
ĒŲĘÔČ·ķĻÓÐŧúÎïžŨĩÄ―áđđžōĘ―Ģš__________________Ģŧ
ĒÚÓÃŧŊŅ§·―ģĖĘ―ąíĘūÏÂÁÐŨŠŧŊđýģĖĢš
žŨĢŦNaOH![]() ________________________________________________________Ģŧ
________________________________________________________Ģŧ
BĢŦAg(NH3)2OHĻDĄú_______________________________________________________ĄĢ
(2)ČôBēŧÄÜ·ĒÉúŌøūĩ·īÓĶĢŽĮëŧØīðÏÂÁÐÎĘĖâĢš
ĒŲĘÔČ·ķĻAĩÄ―áđđžōĘ―Ģš_______________________________________________________ĄĢ
ĒÚÓÃŧŊŅ§·―ģĖĘ―ąíĘūÏÂÁÐŨŠŧŊđýģĖĢš
žŨĢŦNaOH![]() __________________________________________________Ģŧ
__________________________________________________Ģŧ
AĻDĄúBĢš_________________________________________________Ģŧ
DĻDĄúEĢš___________________________________________________ĄĢ
ēéŋīīð°ļšÍ―âÎö>>
ŋÆÄŋĢšļßÖÐŧŊŅ§ ĀīÔīĢš ĖâÐÍĢš
ĄūĖâÄŋĄŋÏÂÁÐĘĩŅé―áđûēŧÄÜŨũΊÏāÓĶķĻÂÉŧōÔĀíĩÄÖĪūÝÖŪŌŧĩÄĘĮĢĻ°Ē·üžÓĩÂÂÞķĻÂÉĢšÔÚÍŽÎÂÍŽŅđÏÂĢŽÏāÍŽĖåŧýĩÄČΚÎÆøĖ嚎ÓÐÏāÍŽĘýÄŋĩÄ·ÖŨÓĢĐ
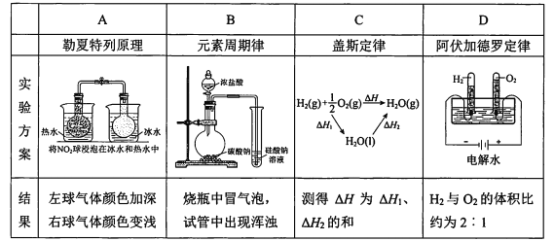
A. AB. BC. CD. D
ēéŋīīð°ļšÍ―âÎö>>
ŋÆÄŋĢšļßÖÐŧŊŅ§ ĀīÔīĢš ĖâÐÍĢš
ĄūĖâÄŋĄŋÏÂÁзīÓĶžČēŧĘôÓÚŅõŧŊŧđÔ·īÓĶĢŽÓÖĘĮÎüČČ·īÓĶĩÄĘĮĢĻ ĢĐ
A. ÂÁÆŽÓëÏĄŅÎËáĩÄ·īÓĶ B. Ba(OH)28H2OÓëNH4ClĩÄ·īÓĶ
C. ÉúĘŊŧŌÓëËŪ·īÓĶ D. žŨÍéÔÚŅõÆøÖÐĩÄČžÉÕ·īÓĶ
ēéŋīīð°ļšÍ―âÎö>>
ŋÆÄŋĢšļßÖÐŧŊŅ§ ĀīÔīĢš ĖâÐÍĢš
ĄūĖâÄŋĄŋĮë―Ŧ·ûšÏĖâŌâĩÄÏÂÁÐąäŧŊĩÄÐōšÅĖîÔÚķÔÓĶĩÄšáÏßÉÏĢšĒŲĩâĩÄÉýŧŠĢŧĒÚŅõÆøČÜÓÚËŪĢŧĒÛÂČŧŊÄÆČÜÓÚËŪĢŧĒÜÉÕžîČÛŧŊĢŧĒÝÂČŧŊĮâČÜÓÚËŪĢŧĒÞÂČŧŊï§ĘÜČČ·Ö―âĄĢ
ĢĻ1ĢĐŧŊŅ§žüÃŧÓÐąŧÆÆŧĩĩÄĘĮ__________Ģŧ―ö·ĒÉúĀëŨÓžüÆÆŧĩĩÄĘĮ________Ģŧ
ĢĻ2ĢОȷĒÉúĀëŨÓžüÆÆŧĩĄĒÓÖ·ĒÉúđēžÛžüÆÆŧĩĩÄĘĮ______________Ģŧ
ĢĻ3ĢĐNa2O2ĩÄĩįŨÓĘ―ÎŠ________Ģŧ
ĢĻ4ĢĐÓÃĩįŨÓĘ―ąíĘūMgCl2ĩÄÐÎģÉđýģĖ _____________
ēéŋīīð°ļšÍ―âÎö>>
ŋÆÄŋĢšļßÖÐŧŊŅ§ ĀīÔīĢš ĖâÐÍĢš
ĄūĖâÄŋĄŋÓëÔŠËØÔÚÖÜÆÚąíÖÐĩÄÎŧÖÃŋÏķĻÎÞđØĩÄĘĮĢĻ ĢĐ
A. ÔŠËØĩÄÔŨÓÐōĘý B. ÔŨÓĩÄšËĩįšÉĘý C. ÔŨÓĩÄÖĘŨÓĘý D. ÔŨÓšËÄÚĩÄÖÐŨÓĘý
ēéŋīīð°ļšÍ―âÎö>>
ŋÆÄŋĢšļßÖÐŧŊŅ§ ĀīÔīĢš ĖâÐÍĢš
ĄūĖâÄŋĄŋÏÂÁÐÓÐŧúÎïĘôÓÚĖþĩÄĘĮ(ĄĄĄĄ)
A.C3H8
B.C2H5OH
C.CH2Cl2
D.NO2
ēéŋīīð°ļšÍ―âÎö>>
ŋÆÄŋĢšļßÖÐŧŊŅ§ ĀīÔīĢš ĖâÐÍĢš
ĄūĖâÄŋĄŋŌŅÖŠŧŊŅ§·īÓĶĒŲ:Fe(s)+CO2(g)![]() FeO(s)+CO(g),ÆäŧŊŅ§Æ―šâģĢĘýΊK1;ŧŊŅ§·īÓĶĒÚ:Fe(s)+H2O(g)
FeO(s)+CO(g),ÆäŧŊŅ§Æ―šâģĢĘýΊK1;ŧŊŅ§·īÓĶĒÚ:Fe(s)+H2O(g)![]() FeO(s)+H2(g),ÆäŧŊŅ§Æ―šâģĢĘýΊK2,ÔÚÎÂķČ973 KšÍ1173 KĩÄĮéŋöÏÂ,K1ĄĒK2ĩÄÖĩ·ÖąðČįÏÂ:
FeO(s)+H2(g),ÆäŧŊŅ§Æ―šâģĢĘýΊK2,ÔÚÎÂķČ973 KšÍ1173 KĩÄĮéŋöÏÂ,K1ĄĒK2ĩÄÖĩ·ÖąðČįÏÂ:
ÎÂķČ | K1 | K2 |
973 K | 1.47 | 2.38 |
1 173 K | 2.15 | 1.67 |
(1)ÍĻđýąíļņÖÐĩÄĘýÖĩŋÉŌÔÍÆķÏ:·īÓĶĒŲĘĮ_______(ĖÎüČČĄąŧōĄ°·ÅČČĄą)·īÓĶĄĢ
(2)ÏÖÓзīÓĶĒÛ:CO2(g)+H2(g)![]() CO(g)+H2O(g),ĮëÄãÐīģöļ÷īÓĶĩÄÆ―šâģĢĘýK3ĩÄąíīïĘ―:K3=______ĄĢ
CO(g)+H2O(g),ĮëÄãÐīģöļ÷īÓĶĩÄÆ―šâģĢĘýK3ĩÄąíīïĘ―:K3=______ĄĢ
(3)ļųūÝ·īÓĶĒŲÓëĒÚŋÉÍÆĩžģöK1ĄĒK2ÓëK3ÖŪžäĩÄđØÏĩĘ―ÎŠ__________,ūÝīËđØÏĩĘ―ž°ÉÏąíĘýūÝ,ÄÜÍÆķÏģö·īÓĶĒÛĘĮ________(ĖÎüČČĄąŧōĄ°·ÅČČĄą)·īÓĶĄĢ
(4)ŌŠĘđ·īÓĶĒÛÔÚŌŧķĻĖõžþÏÂ―ĻÁĒĩÄÆ―šâÏōÕý·īÓĶ·―ÏōŌÆķŊ,ŋÉēÉČĄĩÄīëĘĐÓÐ______ ĄĒ_____ (ĖîÐīŨÖÄļÐōšÅ)ĄĢ
A.ËõÐĄ·īÓĶČÝÆũĩÄČÝŧý B.ĀĐīó·īÓĶČÝÆũĩÄČÝŧý
C.ÉýļßÎÂķČ D.ĘđÓÚÏĘĘĩÄīßŧŊžÁ
E.Éč·ĻžõÐĄÆ―šâĖåÏĩÖÐĩÄCOĩÄÅĻķČ
(5)ÍžžŨĄĒŌŌ·ÖąðąíĘū·īÓĶĒÛÔÚt1ĘąŋĖīïĩ―Æ―šâ,ÔÚt2ĘąŋĖŌōļÄąäÄģļöĖõžþķø·ĒÉúąäŧŊĩÄĮéŋö:
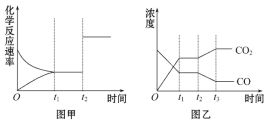
ĒŲÍžžŨÖÐt2ĘąŋĖ·ĒÉúļÄąäĩÄĖõžþĘĮ__________ĄĢ
ĒÚÍžŌŌÖÐt2ĘąŋĖ·ĒÉúļÄąäĩÄĖõžþĘĮ__________ĄĢ
ēéŋīīð°ļšÍ―âÎö>>
ŋÆÄŋĢšļßÖÐŧŊŅ§ ĀīÔīĢš ĖâÐÍĢš
ĄūĖâÄŋĄŋÏÂÁÐÎïÖĘĩÄÓÃÍūēŧÕýČ·ĩÄĘĮ( )
A.ÐĄËÕīōŋÉŨũÖÆļâĩãĩÄ·Ē―ÍžÁ
B.ū§ĖåđčŋÉÓÃÓÚÖÆđâĩžÏËÎŽ
C.ÄÆŋÉÓÃÓÚÖÆļßŅđÄÆĩÆ
D.ŅõŧŊĖúģĢÓÃÓÚšėÉŦÓÍÆášÍÍŋÁÏ
ēéŋīīð°ļšÍ―âÎö>>
đúžĘŅ§ÐĢÓÅŅĄ - Á·Ï°ēáÁÐąí - ĘÔĖâÁÐąí
šþąąĘĄŧĨÁŠÍøÎĨ·ĻšÍēŧÁžÐÅÏĒūŲąĻÆ―ĖĻ | ÍøÉÏÓКĶÐÅÏĒūŲąĻŨĻĮø | ĩįÐÅÕĐÆūŲąĻŨĻĮø | ÉæĀúĘ·ÐéÎÞÖũŌåÓКĶÐÅÏĒūŲąĻŨĻĮø | ÉæÆóĮÖČĻūŲąĻŨĻĮø
ÎĨ·ĻšÍēŧÁžÐÅÏĒūŲąĻĩįŧ°Ģš027-86699610 ūŲąĻÓĘÏäĢš58377363@163.com