
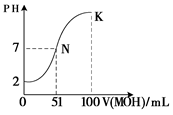
 CH3COOH + OH?��
CH3COOH + OH?�� CH3COOH + OH?�����ݵ���غ���Եõ�c��H+��+c��Na+��=c��OH?��+c��CH3COO?������Ϊ��Һ�Լ��ԣ�����c��OH-����c��H+��������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊc��Na+����c��CH3COO?����c��OH-����c��H+������4��HClΪǿ�ᣬ������Ϊ���ᣬ���Խ���PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���mС��n����5����ͼ����֪���� 0.01 mol��L��1HA��ҺpHΪ2������HAΪǿ��� K���Ӧ����Һ�У����������غ����֪�������MOH�����ʵ���Ϊ0.02��0.1=0.002mol������c(M��)��c(MOH)=0.002/0.2=0.01mol��L��1��
CH3COOH + OH?�����ݵ���غ���Եõ�c��H+��+c��Na+��=c��OH?��+c��CH3COO?������Ϊ��Һ�Լ��ԣ�����c��OH-����c��H+��������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊc��Na+����c��CH3COO?����c��OH-����c��H+������4��HClΪǿ�ᣬ������Ϊ���ᣬ���Խ���PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���mС��n����5����ͼ����֪���� 0.01 mol��L��1HA��ҺpHΪ2������HAΪǿ��� K���Ӧ����Һ�У����������غ����֪�������MOH�����ʵ���Ϊ0.02��0.1=0.002mol������c(M��)��c(MOH)=0.002/0.2=0.01mol��L��1��

 ���������ν�ϵ�д�
���������ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ڷֱ�ϡ����ͬ��������Һ��pH����>�� |
| B����������ѵ����ˮ���ӵ���Ŀ��� |
| C��������ۻ�Ϻ���Һ�����ԣ����ڳ�����Ka��CH3COOH��=Kb��NH3��H2O�� |
| D������ܻ��������Һ�����ԣ���c��CH3COO����+c��OH����<c��CH3COOH��+c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H����A2���ش��������⣺
H����A2���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ��25���80��ʱ��pH |
| B��pH��Ϊ2��������Һ�������е�c��H+�� |
| C��25��ʱ��0.2 mol/L��0.1 mol/L�����ִ�����Һ�д���ĵ���̶� |
| D��25��ʱ���������pH������5�������A1C13����Һ�У��ѵ����ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ͬŨ�ȵ�����Һ��c(OH��) ��ͬ |
| B��pH��13������Һϡ��100����pH��Ϊ11 |
| C��100 mL 0.1 mol/L������Һ���к͵����ʵ��������� |
| D������Һ�зֱ����������Ӧ�������Σ�c(OH��) �����Լ�С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���÷�̪��ָʾ��ʱ�ζ����ܷ�ӦΪ��Na2CO3+HCl=NaHCO3+NaCl |
| B���ü�����ָʾ��ʱ�ζ����ܷ�ӦΪ��Na2CO3+2HCl=NaCl+CO2��+H2O |
| C�����ü�ʽ�ζ�����ȡ����Ҫ��Na2CO3��Һ |
| D������ʽ�ζ���û���ñ���Һ��ϴ��������õ�̼������ҺŨ��ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3OH��l��+3/2O2��g��="=" CO2��g��+ 2H2O��l����H= ��726.4 kJ/mol |
| B��2CH3OH��l��+3O2��g��="=" 2CO2��g��+ 4H2O��g����H= ��1452.8 kJ/mol |
| C��2CH3OH��l��+3O2��g��="=" 2CO2��g��+ 4H2O��l����H= ��726.4 kJ/mol |
| D��2CH3OH��l��+3O2��g��="=" 2CO2��g��+ 4H2O��g����H=" +" 1452.8 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
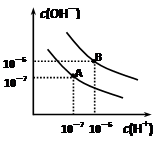
 =1��10-8������������ȷ���ǣ� ����
=1��10-8������������ȷ���ǣ� �����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
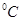 ʱˮ�ĵ���ƽ������ӦΪ ���A����B������
ʱˮ�ĵ���ƽ������ӦΪ ���A����B������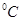 ʱ����
ʱ����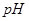 ��8��NaOH��Һ��
��8��NaOH��Һ��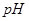 ��5��
��5��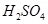 ��Һ��ϣ������û����Һ��
��Һ��ϣ������û����Һ��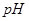 ��7����NaOH��Һ��
��7����NaOH��Һ��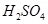 ��Һ�������Ϊ ��
��Һ�������Ϊ ��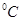 ʱ��0.1 mol/L��NaOH��Һ��pHֵ�� ��
ʱ��0.1 mol/L��NaOH��Һ��pHֵ�� ��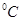 ʱ����100���
ʱ����100���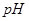 1��
1��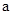 ��ijǿ����Һ��1���
��ijǿ����Һ��1���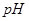 2��b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ��
2��b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ��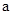 ��b֮��Ӧ����Ĺ�ϵ��
��b֮��Ӧ����Ĺ�ϵ��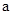 = ���ú�b�Ĵ���ʽ��ʾ��,a+b_______14���<������=����>������
= ���ú�b�Ĵ���ʽ��ʾ��,a+b_______14���<������=����>�������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com