
����Ŀ�����������������������γ����������꣬�������γɹ⻯ѧ�������Ժ��е�������ķ������д�����
(1)������������Һ�������շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�
NO2��NO��2NaOH===2NaNO2��H2O��
2NO2��2NaOH===NaNO2��NaNO3��H2O��
�ڷ�Ӧ���У���������________________����ԭ����________________���ڷ�Ӧ���У��������ͻ�ԭ�������ʵ���֮��Ϊ___________________________________________��
(2)����β���к���CO��NO���������������ʶԴ�������Ⱦ�ķ����ǰ�װ��ת������ʹ���Ƿ�����Ӧ����N2��CO2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
(3)�ɷ�Ӧ���ж϶��������Ƿ�Ϊ����������(������������������)________��ԭ����________________________________________________________________________��
(4)Ŀǰ��һ��������������һ�������£��ð�������������ת��Ϊ����Ⱦ�����ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________���÷�Ӧ�У���������________������������________������1.4 mol����ʱ��ת�Ƶ��ӵ����ʵ���Ϊ_______________________________________________��
(5)Ϊ�˱�����������ҵ�ϳ�ͨ��NH3ʹ����������Ͱ�ת��Ϊ����N2������NO2��NO�Ļ������3 L��ͨ��3 L(ͬ��ͬѹ��)NH3��ǡ��ʹ����ȫת��ΪN2����ԭ���������NO2��NO�����ʵ���֮��Ϊ______��
���𰸡�NO2 NO 1��1 2NO��2CO![]() N2��2CO2 ���� ��Ϊ����������Ӧ���������Σ��ҵ�Ԫ�ػ��ϼ۷����仯 6NO2��8NH3
N2��2CO2 ���� ��Ϊ����������Ӧ���������Σ��ҵ�Ԫ�ػ��ϼ۷����仯 6NO2��8NH3![]() 7N2��12H2O NO2 N2 4.8 mol 1��1
7N2��12H2O NO2 N2 4.8 mol 1��1
��������
(1)������ԭ��Ӧ���������õ��ӻ��ϼ۽��ͣ���ԭ��ʧ���ӻ��ϼ����ߣ�
(2)���ݵ����غ��Ԫ���غ���ƽ����ʽ��
(3)�÷�Ӧ�ж����������ϼ۷����仯��
(4)����ԭ���غ㶨�ɽ����Ŀ��Ϣ��֪�������Ͷ���������Ӧ�����ɵ�����Ⱦ������Ϊ������ˮ��
(5)���ݵ����غ���м��㡣
(1)�ڷ�Ӧ����NO2�еĵ�Ԫ�ػ��ϼ۽�������������NO�е�Ԫ�ػ��ϼ���������ԭ�����ڷ�Ӧ�����������ͻ�ԭ������NO2�����ǵ����ʵ���֮��Ϊ1��1���ʴ�Ϊ��NO2��NO��1��1��
(2)����ԭ���غ㶨�ɽ����Ŀ��Ϣ��֪��һ��������һ����̼��Ӧ�����ɵĶԴ�������Ⱦ������ӦΪ�����Ͷ�����̼�����ݵ����غ��Ԫ���غ�ɵ÷���ʽΪ��2NO��2CO![]() N2��2CO2���ʴ�Ϊ��2NO��2CO
N2��2CO2���ʴ�Ϊ��2NO��2CO![]() N2��2CO2��
N2��2CO2��
(3)����������Ӧ���������Σ��ҵ�Ԫ�ػ��ϼ۷����仯������������������������ʴ�Ϊ�����ǣ���Ϊ����������Ӧ���������Σ��ҵ�Ԫ�ػ��ϼ۷����仯��
(4)����ԭ���غ㶨�ɽ����Ŀ��Ϣ��֪�������Ͷ���������Ӧ�����ɵ�����Ⱦ������Ϊ������ˮ��NO2�У�4�۵�N��NH3�У�3�۵�N��ԭΪN2��0�۵�N��ʣ�µ��⡢��Ԫ�ؽ�ϳ�ˮ�������������NO2�����������ԭ�����ΪN2������7 mol N2ʱ��ת�Ƶ���24 mol��������1.4 mol N2ʱ��ת�Ƶ���4.8 mol���ʴ�Ϊ��6NO2��8NH3![]() 7N2��12H2O��NO2��N2��4.8 mol��
7N2��12H2O��NO2��N2��4.8 mol��
(5) ��NO2�����Ϊx����NO�����Ϊ(3 L��x)��NO2![]()
![]() N2��NO
N2��NO![]()
![]() N2��NH3
N2��NH3![]()
![]() N2�����ݵ�ʧ�����غ�ԭ���ã�
N2�����ݵ�ʧ�����غ�ԭ���ã�![]() �����x��1.5 L����Ϊͬ������������ʵ���֮�ȵ��������֮�ȣ�����n(NO2)��n(NO)��1��1���ʴ�Ϊ��1:1��
�����x��1.5 L����Ϊͬ������������ʵ���֮�ȵ��������֮�ȣ�����n(NO2)��n(NO)��1��1���ʴ�Ϊ��1:1��


 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ֲ���纣���������к��зḻ�ĵ�Ԫ�ء���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�

(1)ָ���Ӻ�������ȡI2��ʵ��������ƣ�
��____________����__________�ڵ����ӷ���ʽ__________����
(2)��ȡ��Ĺ����У��ɹ�ѡ����л��ܼ�����____����
A �ױ����ƾ� B ���Ȼ�̼����
C ���͡����� D ���͡�����
(3)Ϊʹ������I��ת��Ϊ����л���Һ��ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������ȱ�ٵ�������________��
(4)�Ӻ�����л��ܼ�����ȡ�⣬��Ҫ��������ָ����������װ���еĴ���֮��__________��
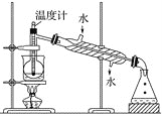
(5)�����������ʱ��ʹ��ˮԡ���ȵ�ԭ����_________________�����̬����________�С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʾ��Һ��Ũ�ȵķ���ͨ�������֣���Һ�����ʵ���������(w)�����ʵ���Ũ��(c)�������������Һʱ�����ݲ�ͬ����Ҫ���в�ͬ�����Ʒ������������ա�
(1)��10%(�ܶ�Ϊ1.01 g��cm��3)������������Һ���Ƴ�27.5 g 2% ������������Һ��
�ټ��㣺��________g 10%(�ܶ�Ϊ1.01 g��cm3)������������Һ�������Ϊ________mL�����________mLˮ(��ˮ��1 g��cm��3)����ϡ�͡�
����ȡ����________mL��Ͳȡ10% �������ƣ���ȡʱ����Ҫ����Ͳ________����ˮƽ��Ȼ�����ձ����________mL��Ͳ��ȡ����ˮҲע���ձ��
���ܽ⣺��________��������Һ������ȣ�����27.5 g 2% ������������Һ��
(2)��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ����ϡ�ͳ�3 mol��L��1��ϡ����100 mL���ش��������⣺
����ҪȡŨ����________mL��
�����Ʋ����ɷֽ�����¼�����������ȷ�IJ���˳����__________________________(����ĸ����ͬ)��
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C��������ȴ��ϡ����ע���Ѽ�鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹ��Һ����ǡ����̶�������
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���
(3)ʵ����������1 mol��L��1������������Һ��1 mol��L��1��������Һ��100mL��
��Ҫ��������������Һ������������ƽ��ȡ�������ƹ���ʱ����ƽ����Ϊ________��
A��4.0 g����������B��4.00 g�������� C����4.0 g
������������������Һ��������Һ�ĸ��������У������Բ�ͬ����__________��
A����������ȡ������B���ܽ��ϡ�� ��C����Һ��ϴ�ӡ���D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�����ӷ���ʽ��ȷ����
A.��Ca(ClO)2��Һ��ͨ�����CO2�ƴ����2ClO + H2O + CO2=2HClO +![]()
B.[Ag(NH3)2]OH���Ũ���ᷴӦ����AgCl��[Ag(NH3)2]+ + OH + 3H+ + Cl=AgCl��+2![]() + H2O
+ H2O
C.Cl2���ȵ�NaOH��Һ��Ӧ��ȡNaClO3��2Cl2 + 6OH![]() 3Cl +
3Cl +![]() + 3H2O
+ 3H2O
D.������KMnO4��Һ��ͨ��SO2��2![]() + 5SO2 + 4OH=2Mn2+ + 5
+ 5SO2 + 4OH=2Mn2+ + 5![]() + 2H2O
+ 2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Դ�����ˮ�����Ⱦ�ǻ�����������Ҫ�о����⡣
(1) ��ѧ�ϲ���NH3����NxOy��������������Ⱦ��������Ϊ��ҵ������������Դ��
��֪��2NO(g)=N2(g)+O2(g) ��H=��177kJ/mol
4NH3(g)+3O2(g)===2N2(g)+6H2O(g) ��H=��1253.4kJ/mol
����NH3����NO���ɵ�������̬ˮ���Ȼ�ѧ����ʽΪ___________________��
(2)��֪��N2(g)+3H2(g) ![]() 2NH3(g) ��H<0����ͬ�¶��£������������зֱ�Ͷ����ͬ���ķ�Ӧ����з�Ӧ����ò�ͬѹǿ��ƽ��������NH3�����ʵ���������ͼ��ʾ��
2NH3(g) ��H<0����ͬ�¶��£������������зֱ�Ͷ����ͬ���ķ�Ӧ����з�Ӧ����ò�ͬѹǿ��ƽ��������NH3�����ʵ���������ͼ��ʾ��

��M���v��_________Q���v��(����>����<������=��)��
��T3�¶��£���1molN2��3molH2����2L���ܱ������У�ά��ѹǿΪ60MPa���䣬�ﵽN���ƽ��״̬����Ӧ��Ũ��ƽ�ⳣ��K=_____________ (����������ʾ)��M���ƽ�ⳣ����N���ƽ�ⳣ��_________(����������С�����������)��
(3)ˮ���й�������(��NH3��ʾ)�ᵼ��ˮ�帻Ӫ������
���ô������Ƴ�ȥ������ԭ����ͼ��ʾ��д���ܷ�Ӧ��ѧ����ʽ��_____________��


��ȡһ�����ĺ�������ˮ���ı����������Ƶ���������Ӧһ��ʱ�����Һ�а���ȥ���ʡ��ܵ�(��Һ�����п����Եĺ����������е�Ԫ�ص�����)ȥ�����Լ�ʣ��������Ƶĺ�����m(NaClO)��m(NH3)�ı仯�������ͼ��ʾ����Bʣ��NaClO�������ڵ�A��ԭ����____����m(NaClO)��m(NH3)>7.6ʱ��ˮ�����ܵ�ȥ���ʷ����½������ܵ�ԭ����__________��
(4)�缫����Ĥ��������ǵ绯ѧ�����﹤�յ���ϡ�ij����Ĥ�����õ������Ļ���ԭ�ӽ�NO3-��ԭΪN2������ԭ������ͼ��ʾ�����������ɱ�״����2.24 L���壬�����Ͽɳ�ȥNO3-�����ʵ���Ϊ_____mol��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������(FeC2O4��2H2O��M=180g/mol)�ʵ���ɫ,������ɹ����ͼ��ijʵ��С����������һϵ��̽����
I.�����������������ȷֽ�����̽��
(1)�������ɷֵ�̽����С���Ա��������װ�ÿ��ظ�ѡ��)����ʵ�飺

��E��ʢװ��ʯ�ҵ���������Ϊ_________��
�ڰ������������ҵķ�������װ�õĽӿ�˳��Ϊa��g��f��_____��β������װ��(�������ظ�ʹ��)��
��ʵ��ǰ��ͨ��һ��ʱ��N2����Ŀ��Ϊ__________________��
��ʵ��֤������������к���CO�����ݵ�ʵ������Ϊ_____________��
(2)С���Ա���ʵ��֤����A�зֽ��Ĺ���ɷ�ΪFeO���������������ֽ�Ļ�ѧ����ʽΪ____________________��
(3)ɹ����ͼʱ����K3[Fe(CN)6]��ҺΪ��ɫ�����÷�Ӧ�Ļ�ѧ����ʽΪ______________��
��.��������������Ʒ���ȵIJⶨ
��ҵ�ƵõIJ������������г�����FeSO4���ʣ��ⶨ�䴿�ȵIJ������£�
����1����ȡmg��������������Ʒ������ϡHSO4�У����250mL��Һ��
����2��ȡ������Һ25.00mL���� cmol/L KMnO4��Һ�ζ����յ㣬���ı�ҺV1mL��
����3����Ӧ����Һ�м�������п�ۣ���ַ�Ӧ��������ϡH2SO4������ cmol/L KMnO4����Һ�ζ����յ㣬���ı�ҺV2mL��
(4)����2�еζ��յ������Ϊ______________������3�м���п�۵�Ŀ��Ϊ_______��
(5)��������������Ʒ�Ĵ���Ϊ________��������1������Һʱ����Fe2+���������ʣ���ⶨ�����____(����ƫ��������ƫ��������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ̽��������������ʣ�����ͼ��ʾװ�ý���ʵ�顣

��ش��������⣺
��1��װ��A��ʢ���������Ƶ�����������__________����Ӧ�Ļ�ѧ����ʽΪ_______________��
��2��װ��B�е�������__________________����Ӧ�����ӷ���ʽΪ_________________��
��3��װ��C�е�������____________________��������˵������������е�������________________________��
��4��װ��D��Ŀ����̽������������Ʒ�����õĿ����ԣ�д��ʵ�����������_____________��
��5��β���ɲ���__________��Һ���ա�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.�о�������ʴ�ͷ�����ԭ��������ʵ���塣
(1)��ͼΪ�˽̰�̲���̽��������������ʴ��װ�á�ij��ȤС�鰴��װ��ʵ�飬������Һ�����������������д�ʩ���Ը���������۲쵽ˮ�������������______(�����)��
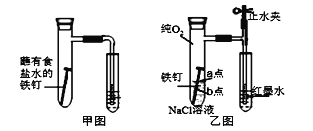
A.�ô����������Թ��ڿ��� B.�þƾ��Ƽ����Թ�����¶�
C.�������������ۺ�̿�ۻ�Ϸ�ĩ D.���ɸ�ϸ�ĵ��ܣ�ˮ�еμӺ�īˮ
(2)��С�齫��ͼװ�øĽ�����ͼװ�ò�����ʵ�飬�����к�īˮҺ���߶���ʱ��ı仯���±������������жϸ�ʴ��������ʱ����______(�����ӿ�����������������������)������ΪӰ������Ϊ_______��
ʱ��/min | 1 | 3 | 5 | 7 | 9 |
Һ���߶�/cm | 0.8 | 2.1 | 3.0 | 3.7 | 4.2 |
(3)Ϊ̽��������ʴʵ�� a��b �����������ķ�Ӧ����������ʵ�飬����ɱ���հף�
ʵ����� | ʵ������ | ʵ����� |
��NaCl��Һ�еμ�2~3�η�ָ̪ʾ�� | a�㸽����Һ���ֺ�ɫ | a��缫��ӦΪ_____ |
Ȼ���ٵμ�2~3�����軯����Һ | b����Χ������ɫ���� | b��缫��ӦΪ Fe -2e-=Fe2+ |
(4)�������װ���о������Ի����и�ʴ����Ҫ��ʽ���ⶨ��ƿ����ѹ�Ϳ��������������������ʱ��仯��ͼ����ͼ�пɷ�����t1~t2֮����Ҫ����_______��ʴ(������������)��ԭ��_______��
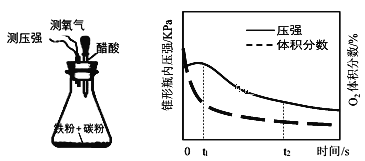
(5)���������ۻ���һ�ֵ绯ѧ������������Fe����������H2SO4��Һ�У�һ��������Fe�ۻ��γ�����Fe3O4����Ĥ����д���������缫��Ӧʽ______��
II.��֪���ᾧ��(H2C2O4��XH2O)������ˮ�����������Ը��������Һ��ȫ��Ӧ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O������������ԭ�ζ����ⶨ���ᾧ��Ľᾧˮ������X���������£�
���÷�����ƽ��ȡ���ᾧ��1.260g���������Ƴ�100.00mL���������Һ
������Һ����ȡ25.00mL���������Һ����ƿ�У����������������ữ
����Ũ��Ϊspan>0.1000mol/L��KMnO4 ����Һ���еζ������ν�����£�
��һ�εζ� | �ڶ��εζ� | �����εζ� | |
������Һ���(mL) | 25.00 | 25.00 | 25.00 |
����Һ���(mL) | 9.99 | 10.01 | 10.00 |
��֪H2C2O4����Է�������Ϊ90����ش��������⣺
(1)�ζ�ʱ��KMnO4����ҺӦ��װ��______(������ʽ��������ʽ��)�ζ����С�
(2)����ζ��յ�ı�־��________��
(3)�����������ݼ���X=_______��
(4)������(��ƫ�ߡ�ƫ�ͻ���Ӱ��)�����ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ���Xֵ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л��������ڶ࣬�ֲ����㣬�������ϵ�dz����С�
��1��ʯ���ѽ�õ�ij��A�������ģ��Ϊ![]() ��������Ҫ�Ļ�������ԭ�ϡ�
��������Ҫ�Ļ�������ԭ�ϡ�
��A�Ľṹ��ʽΪ_________��A��������____________��
��A��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽΪ_______________��

��A��C�ķ�Ӧ������____��C+D��E�Ļ�ѧ����ʽΪ_______������C��D�ķ�����_______��
��A��ͬϵ��B����Է���������A��14��B�Ľṹ��____�֡�
��2����ƻ��������Ʊ�������ƻ��֭����������Һ��Ӧ��ƻ������������ʱ��������ط�Ӧ����ʽΪ__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com