
����Ŀ����֪����ʮ�����ʣ�
��H2O����Cu����NO����SiO2����ϡ���ᡡ�������������߱���FeCl3��Һ���ఱˮ����ϡ���ᡡ��������
���������ṩ�����ʣ��ش��������⣺
��1�����ڴ��������__________�����ڵ���ʵ���__________��(���������)
��2�������кͷ�Ӧ�����ӷ���ʽΪH����OH��===H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽ��__________________________________��
��3��ʵ�����Ʊ�����Fe(OH)3�������õ���������________(���������)��������Ӧ�����ӷ���ʽΪ_________________________��
��4��ʵ��������0.5 mol��L��1 245 mL�����Һ����Ҫ�õ��IJ��������в��������ձ�����ͷ�ιܡ�________����Ҫ��������ƽ��ȡ���ʵ�����Ϊ________g�������ƺõ���Һ��ȡ��100 mL�����к��е�SO42����ĿΪ________(��NAΪ�����ӵ�������ֵ)��
���𰸡� �٢ڢۢܢޢ� �٢ޢ� Ba(OH)2��2HNO3===Ba(NO3)2��2H2O �٢� Fe3����3H2O![]() 3H����Fe(OH)3(����) 250 mL����ƿ 42.8 0.15NA
3H����Fe(OH)3(����) 250 mL����ƿ 42.8 0.15NA
����������1��H2O��Cu��NO��SiO2���������������������ڴ�����ʴ�Ϊ�٢ڢۢܢޢ⣻���ʺͻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ����������ˮ��Һ�л�����״̬���ܵ���Ļ����H2O���������������������ڵ���ʣ��ʴ�Ϊ�٢ޢ⣻��2��Ba(OH)2��2HNO3��Ӧ��ʵ����H����OH��=H2O����ϡ������Ba(OH)2��Ӧ��������ˮ�����������ᱵ�����������ϡ��ʷ����кͷ�Ӧ�����ӷ���ʽΪH����OH��=H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽ��Ba(OH)2��2HNO3=Ba(NO3)2��2H2O����3��ʵ�����Ʊ�����Fe(OH)3�������õ�������������ˮ�ͱ���FeCl3��Һ����ѡ�٢ߣ�������Ӧ�����ӷ���ʽΪ��Fe3����3H2O![]() 3H����Fe(OH)3(����)����4��û��245 mL��������ƿ����ʵ��������0.5 mol��L��1 245 mL��������Һ����������0.5 mol��L��1 250 mL��������Һ��0.5 mol��L��1 245 mL��������Һ�IJ����У����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫ�������У�������ƽ��ҩ�ס���Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ����Ի���Ҫ�IJ�������Ϊ��250mL����ƿ����Ҫ��������ƽ��ȡ���ʵ�����m[Al2(SO4)3]= 0.5 mol��L��1��0.25L��342g/mol=42.8g��100 mL����Һ�к��е�SO42����ĿΪ0.5 mol��L��1��0.10L��3��NAmol-1=0.15NA��
3H����Fe(OH)3(����)����4��û��245 mL��������ƿ����ʵ��������0.5 mol��L��1 245 mL��������Һ����������0.5 mol��L��1 250 mL��������Һ��0.5 mol��L��1 245 mL��������Һ�IJ����У����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫ�������У�������ƽ��ҩ�ס���Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ����Ի���Ҫ�IJ�������Ϊ��250mL����ƿ����Ҫ��������ƽ��ȡ���ʵ�����m[Al2(SO4)3]= 0.5 mol��L��1��0.25L��342g/mol=42.8g��100 mL����Һ�к��е�SO42����ĿΪ0.5 mol��L��1��0.10L��3��NAmol-1=0.15NA��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�������ǵ绯ѧʵ��װ����
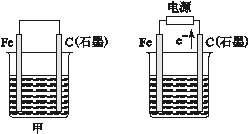 ��
��
��1�������������ձ��о�ʢ��NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ_________________________________________________��
�������ܷ�Ӧ�����ӷ���ʽΪ___________________________________________________��
�۽�ʪ��ĵ���KI��ֽ�������ձ��Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�����������������÷�Ӧ�Ļ�ѧ����ʽΪ_____��
��2��)�����������ձ��о�ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ___________________________________________________��
�������ʼʱ����ʢ��200 mL pH��5��CuSO4��Һ(25 ��)��һ��ʱ�����Һ��pH��Ϊ1����Ҫʹ��Һ�ָ������ǰ��״̬��������Һ�м���_____________________(��д���ʵĻ�ѧʽ)______________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵķ����У��������ۼ����� �� ��
A. NaCl B. Cl2 C. HCl D. NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦX��g����3Y��g��![]() 2Z��g������H��0 �ڲ�ͬ�¶ȡ���ͬѹǿ��P1��P2���£��ﵽƽ��ʱ�����������Z����������գ�Z�����¶ȱ仯������Ϊ ( )
2Z��g������H��0 �ڲ�ͬ�¶ȡ���ͬѹǿ��P1��P2���£��ﵽƽ��ʱ�����������Z����������գ�Z�����¶ȱ仯������Ϊ ( )

A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������й㷺����;�������к�������Fe��Zn��Cu��Pt������,���õ�ⷨ�Ʊ��ߴ��ȵ���������������ȷ����(��֪:������Fe2+<Ni2+<Cu2+)(����)
A. ����,���۵ײ�����������ֻ�н���Pt
B. ��������,�������Һ���������ܱ��ֲ���
C. ����,��Һ�д��ڵĽ���������ֻ��Fe2+��Zn2+
D. ��������������Ӧ,��缫��Ӧʽ:Ni2++2e-![]() Ni
Ni
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. 1H��2H��3H��H+��H2��Ϊͬλ�� B. HDΪ������
C. H2��D2��������ȫ��ͬ D. C60�����ʯ��ʯī��Ϊͬ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
����������(FexNy)�ڴż�¼�����������Ź㷺��Ӧ��ǰ����ijFexNy���Ʊ���������������ͪ���Ҵ����롣
��1��Fe3+��̬��������Ų�ʽΪ____________________��
��2����ͪ(![]() )������̼ԭ�ӹ�����ӻ�������_______________,1 mol ��ͪ�����к��ЦҼ�����ĿΪ______________��
)������̼ԭ�ӹ�����ӻ�������_______________,1 mol ��ͪ�����к��ЦҼ�����ĿΪ______________��
��3��C��H��O ����Ԫ�صĵ縺����С�����˳��Ϊ________________��
��4���Ҵ��ķе���ڱ�ͪ,������Ϊ____________________��
��5��ijFexNy�ľ�������21ͼ-1��ʾ,Cu������ȫ����þ�����aλ��Fe����bλ��Fe,�γ�Cu����Ͳ���Fe(x-n) CunNy��FexNyת��Ϊ����Cu����Ͳ���������仯����21ͼ-2 ��ʾ,���и��ȶ���Cu����Ͳ���Ļ�ѧʽΪ___________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͨ�������£�����ͼ��ʾװ�����Ҷ�ȩ��OHC-CHO���Ʊ��Ҷ��ᣨH00C-COOH�������Ʊ���ӦΪ��OHC-CHO+2Cl2+2H2O��HOOC-COOH+4HCl������˵����ȷ����

A. ÿ����0.1mol�Ҷ�ȩ��Pt1���ų�2.24L���壨��״����
B. Pt1�ĵ缫��ӦΪ��4OH--4e-=2H2O+O2��
C. ÿ�õ�lmol�Ҷ��Ὣ��2molH+������Ǩ�Ƶ�����
D. ���������ṩCl-����ǿ�����Ե�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�������ֵ������������ȷ����
A. 60g�����д��ڵĹ��ۼ�����Ϊ10NA
B. 1L 0��1 mol��L-1��NaHCO3��Һ��HCO3-��CO32-��������֮��Ϊ0��1NA
C. ���ڿ�����ȼ�տ����ɶ��������23g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ1 NA
D. 235g����![]() �����ѱ䷴Ӧ��
�����ѱ䷴Ӧ��![]() +
+![]() +
+![]() +10
+10![]() �������������ӣ�
�������������ӣ�![]() ����Ϊ10 NA
����Ϊ10 NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com