
| 0.4V-0.2V |
| 2V |
| Ksp |
| c(CO32-) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ��ӦX��g��+Y��s��?Z��s��+R��g������H��0���������淴Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ��t1ʱ��ʼ�ı���������ı�������������ߵ��ǣ�������
��ͼ��ʾ��ӦX��g��+Y��s��?Z��s��+R��g������H��0���������淴Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ��t1ʱ��ʼ�ı���������ı�������������ߵ��ǣ�������| A����������Z | B������ |
| C����ѹ | D���ô��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������2cmol?L-1 |
| B������2cmol?L-1 |
| C����2cmol?L-1 |
| D����c��2cmol?L-1֮�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 |  |  |  |
| A������һ�����ʵ���Ũ��������Һ | B���ſ������ռ����� | C����ȡ9.3mlϡ���� | D���Ʊ��������� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
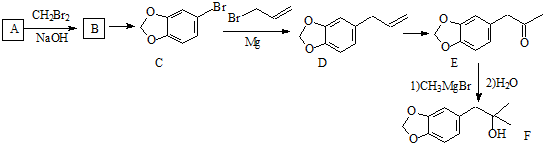
 ϵͳ����Ϊ1-���ϩ��
ϵͳ����Ϊ1-���ϩ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
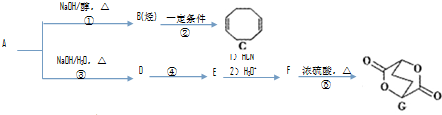

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��431 KJ/mol |
| B��946 KJ/mol |
| C��649 KJ/mol |
| D��869 KJ/mol |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com