
���ֶ�����Ԫ��A��B��C��D��E��ԭ��������������A��Cͬ�壬B��D ͬ�壬C���Ӻ�B���Ӿ�����ͬ�ĵ��Ӳ�ṹ��A��B��D��E�����γɹ����ͻ����A��B�γɵ�������ˮ�гʼ��ԣ�C��E�γɵĻ�������ˮ�г����ԡ��ش��������⣺
��1������Ԫ���У�ԭ�Ӱ뾶������ ���ǽ�������ǿ���� ����Ԫ�ط��ţ���
��2����A��B��D��E���γɵĹ����ͻ������У����ȶ��������� ���û�ѧʽ��ʾ����
��3��A��E�γɵĻ�������A��B�γɵĻ����ﷴӦ������Ļ�ѧʽΪ �����д��ڵĻ�ѧ������Ϊ ��
��4��D����������ˮ����Ļ�ѧʽΪ ��
��5������E��ԭ�ӽṹʾ��ͼΪ ��D�ڲ������E��ȼ�գ����ɵ���Ҫ����Ļ�ѧʽΪ ��
��6������E��ˮ��Ӧ�����ӷ���ʽΪ ��
��1��Na �� Cl
��2��PH3
��3��NH4Cl
��4��H3PO4
��5�� ��PCl3
��PCl3
��6��Cl2+H2O=HClO+H++Cl-
�������������A��B�γɵ�������ˮ�гʼ��ԣ�˵��A��H��B��NԪ�أ�A��B��C��D��E��ԭ������������������C��Na��D��PԪ�أ�C��E�γɵĻ�������ˮ�г����ԣ�E��ClԪ�ء�
��1������Ԫ���У�ԭ�Ӱ뾶������Na���ǽ�������ǿ����Cl��
��2��B��D��E�зǽ�������������P��������A�γɵ���̬�⻯�����ȶ�������PH3
��3��A��E�γɵĻ��������Ȼ��⣬A��B�γɵĻ������ǰ��������߷�Ӧ�����Ȼ�泥���ѧʽΪNH4Cl
(4) D����������ˮ���������ᣬ��ѧʽΪH3PO4
��5���ȵ�ԭ�ӽṹʾ��ͼΪ ��P�ڲ����������ȼ�գ��������Ȼ��ף���ѧʽΪPCl3
��P�ڲ����������ȼ�գ��������Ȼ��ף���ѧʽΪPCl3
��6��������ˮ��Ӧ�����ӷ���ʽΪCl2+H2O=HClO+H++Cl-
���㣺����Ԫ���ƶϣ�ԭ�Ӱ뾶���ǽ����ԡ���̬�⻯����ȶ��ԡ���ѧʽ��ԭ�ӽṹʾ��ͼ�����ӷ���ʽ���ж�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�����ѧ�������ʽṹ�����ʡ�
����Ԫ�ذ���N��P��As��Sb��Bi����Ԫ�ء�
��1�����й��ڵ���Ԫ�ص�˵����ȷ���� ��
| A��N2�����������ơ��䷢��ԭ���ǵ��Ӵ������ϵ͵Ĺ��ԾǨ�������ϸߵĹ�����Թ����ʽ�ͷ����� |
| B��P��Na��S����Ԫ�صĵ�һ�������ɴ�С��˳���ǣ�P>S>Na |
| C����̬Asԭ���У�����ռ�ݵ�����ܼ�Ϊ4d |
| D��Biԭ�����������5��������ͬ�ĵ��� |
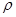 g��cm��3�������ı߳�Ϊacm�����ӵ�����Ϊ__ mol-l���ú��ѡ�a��ʽ�ӱ�ʾ����
g��cm��3�������ı߳�Ϊacm�����ӵ�����Ϊ__ mol-l���ú��ѡ�a��ʽ�ӱ�ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±����г����ֶ�����Ԫ��A��B��C��D��E����Ϣ�����ƶϺ�ش�
| Ԫ�� | �� �� �� Ϣ |
| A | Ԫ����Ҫ���ϼ�Ϊ-2��ԭ�Ӱ뾶Ϊ0.074 nm |
| B | ��������������������������֮��Ϊ4 |
| C | ԭ�Ӱ뾶Ϊ0.102 nm���䵥��Ϊ��ɫ���壬����A�ĵ�����ȼ�� |
| D | ����������ˮ�����ܰ�1�U1�������������ȵ����������� |
| E | ԭ�Ӱ뾶Ϊ0.075 nm������������ˮ����������⻯���γ�һ����X |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F����Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�ء�����֮��Ĺ�ϵ���£�
����ԭ�Ӱ뾶��A �� C �� B �� E �� D
����ԭ�ӵ�������������A �� D�� C �� E�� A��B �� C��
����ԭ�ӵĺ�����Ӳ�����B �� C �� 2A
������BԪ�ص���Ҫ���ϼۣ�������ۣ������ �� 2
������F�ĵ��ʻ�Ͻ����������Ľ������ϡ�
��ش𣺣�1������A��B����Ԫ�ذ�ԭ����֮��Ϊ3:1��ɵ����ʣ�д�������ʽ ��
��2��д��ij��F��ɫ�Ĵ�����������B����������Ӧˮ�����ϡ��Һ��Ӧ�����ӷ���ʽ�� ������ ��
��3����A��C��E����Ԫ����ɵ��������廯������Ӧ�����ɵ���ɫ����Ļ�ѧ��Ӧ����ʽΪ�� ��
��4������Ԫ����A��B��C��E��F������Ԫ�ؿ��γ�һ�ֳ������Σ������ø���������������������Ӹ�����Ϊ1:1:2���������Һ�����ữ��BaCl2��Һ��������ɫ����������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ���������壬��ø��εĻ�ѧʽΪ ��Ϊ����ø����е�ij����ɫ���Ӵ��ڣ���д��ʵ��IJ������������ ��
��5���������������0.1L 0.1mol/L����Һ�������м���0.06mol��BaCl2������ȫ��Ӧ����Һ��c��SO42-��Ϊ�������� ����֪��������Һ����仯���ó�����Ksp=2��10-9��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±���Ԫ�����ڱ���һ���֣��ش������й����⣺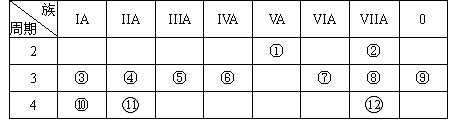
��1��д������Ԫ�ط��ţ��� ���� ���� ��
��2������ԭ�ӵĽṹʾ��ͼ���� ���� ���� ��
��3������ЩԪ���У�����õĽ���Ԫ���� ������õķǽ���Ԫ���� ������õ�Ԫ���� ��������Ԫ�ط��ţ�
��4������ЩԪ�ص�����������Ӧˮ�����У�������ǿ���� ��������ǿ���� �������Ե����������� ��
��5���ڢ�����У���ѧ���ʽϻ��õ��� ����Ԫ�ط��ţ��������û�ѧʵ��֤����
�� ���ÿ�2�֣�����ÿ��1�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I����Ԫ�ط��Żش�ԭ������11-18��Ԫ�ص��й����⣺(���Ӧ���ʵĻ�ѧʽ)
��1������������ˮ���������ǿ���� ;�����ʽΪ��
��2������������ˮ��������Ե��� ;
��3�����γ���̬�⻯�������ȶ����� �������ʽΪ ��
II����ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����������ʵ��֪ʶ�ش��������⡣
����˵��������� ��
| A����Cl2ͨ����ɫʯ����Һ����Һ�ȱ�����ɫ |
| B����Һʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| C������1L0��5mol��L-1��NaCl��Һ������������ӳ���29��25gNaCl���� |
| D�������£�������������������Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
V��W��X��Y��Z��ԭ���������εݼ������ֳ���Ԫ�ء�XԪ���ǵؿ��к�������Ԫ�أ�Y��Z��ɵ���̬������M��ˮ��Һ�ʼ��ԣ�W�ĵ�����X2��ȼ�ղ����ʹƷ����Һ��ɫ��V��һ����ʷ�ƾá�Ӧ�ù㷺�Ľ���Ԫ�ء���ش�
��1��YԪ�������ڱ��е�λ���� ��д��X��Z����Ԫ����ɵĻ�����Z2X2��һ����;�� ��
��2������������Ԫ������������γɵĻ������У���һ����������ˮ��Ӧ���������ҷ�Ӧ����������ԭ��Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��X��Y��Z����Ԫ�ؿ����һ��ǿ��U��M���ʵ������±�U��������һ���Ρ����ε�ˮ��Һ��pH 7������ڡ�����С�ڡ����ڡ�����ԭ���ǣ������ӷ���ʽ��ʾ��
��4������V����Ͷ�뵽�����У�������dz��ɫ��ҺN��N��������Һ��˫��ˮ��Ӧ�����ӷ���ʽ��
��5����������Ѱ����ʵĴ����͵缫���ϣ���Y2��Z2Ϊ�缫��Ӧ���HCl��NH4Cl��ҺΪ�������Һ��������ȼ�ϵ�أ���д���õ�ص������缫��Ӧʽ ���ŵ�ʱ��Һ��H+���� �����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D 4��Ԫ�ص�ԭ��������С��18,�������������Ϊ+1��+4��+5��+7,��֪B��ԭ�Ӻ������������Ϊ2��A��Cԭ�ӵĺ������������Ϊ8��DԪ�ص�����������Ӧˮ��������֪������������ǿ���ᡣ��:
(1)A��B��C��D�ֱ�����������������������������������������
(2)A�����ӽṹʾ��ͼΪ����������,C��ԭ�ӽṹʾ��ͼΪ����������
(3)C������������Ӧˮ������A���������ﷴӦ����������������,�仯ѧʽΪ����������������������������������(�ɲ�����,Ҳ�ɲ���)
(4)C��D����̬�⻯���ȶ�����ǿ������˳������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ����������������֪A��D��C��E
�ֱ�ͬ���壬D��E��Fͬ���ڡ�A��B������������֮����C��������������ȣ�A�ֱ���B��C���γɵ���������ȵķ��ӣ���A��C��A��E��A��FҲ�����γɵ���������ȵķ��ӡ�
��ش��������⣺
��1��Ԫ��F�����ڱ��е�λ��________________________________________________��
��2��A��C��D����Ԫ�ؿ����һ�ֳ���������û�����Ļ�ѧʽΪ________����ҵ�����û�����͵���F�����ӷ���ʽΪ_________________________________________��
��3��B��F�γɵĻ���������У���ԭ����������8���ӽṹ����÷��ӵĵ���ʽΪ________________________________________________________________________��
��4����֪0.50 mol EC2��C2��������̬EC3���ų�49.15 kJ���������Ȼ�ѧ����ʽΪ_________________________________________________________________________��
��5��A��B��C��ԭ�Ӹ�����4��2��3���γɵĻ���������Ļ�ѧ������Ϊ____________��
0��1 mol��L��1�û�����ˮ��Һ�е�����Ũ���ɴ�С��˳��Ϊ_________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com