
����Ŀ��������Ԫ�ص�ҡ������ˮ�к��д���±��Ԫ�ء�NaCl�����ṹʾ��ͼ������ʾ�������߳�Ϊa nm).

(1)Ԫ�� Na�ļ۵��ӱ����������ڸ��ܼ����γɵļ���̬Naԭ�ӣ���۵��ӹ����ʾʽΪ__________��
(2)���� NaCl��Cl Ԫ�ػ����γɶ��ּ�̬�Ļ������NaClO��NaClO2��NaClO3��NaClO4�����������ζ�Ӧ���������������ǿ���Խ���HClO4������ǿ��HClO3��ԭ��__________��
(3)���ʵ������£����NaClˮ��Һ���Ƶ� NaClO3��
��NaClˮ��Һ�д��ڵ�������������________������ţ���
A.���Ӽ� B.���Լ� C.��λ�� D.���
�ڸ��ݼ۲���ӶԻ������ۣ�Ԥ��ClO3-�Ŀռ乹��Ϊ________��д��һ��ClO3-�ĵȵ�����Ļ�ѧ���ţ�______________
(4)��NaCl�����У�Na λ��Cl ��Χ�ɵ���______��������ģ��ö�����ı߳���______nm��
(5)Na �뾶��Cl�뾶�ı�ֵΪ______������С�����3λ��![]() =1.414)��
=1.414)��
���𰸡�![]() HClO3��HClO4���ɷֱ��ʾΪ(HO)ClO2����(HO)ClO3��HClO3��ClΪ+5�ۣ�HClO4��ClΪ+7�ۡ����������Ը��ߣ�����H��O֮��ĵ��Ӷ���Oƫ�ƣ��������H+����HClO4�з��ǻ�������HClO3�࣬��HClO4���Ա�HClO3ǿ�� BCD ������ SO32-��IO3-��BrO3- ��
HClO3��HClO4���ɷֱ��ʾΪ(HO)ClO2����(HO)ClO3��HClO3��ClΪ+5�ۣ�HClO4��ClΪ+7�ۡ����������Ը��ߣ�����H��O֮��ĵ��Ӷ���Oƫ�ƣ��������H+����HClO4�з��ǻ�������HClO3�࣬��HClO4���Ա�HClO3ǿ�� BCD ������ SO32-��IO3-��BrO3- �� ![]() 0.414
0.414
��������
��1��Ԫ��Na����ɫ��Ӧ�ʻ�ɫ������̬Naԭ�Ӽ۵�����3s�ܼ�������3p�ܼ�����۵��ӹ����ʾʽΪ![]() ��
��
��2��HClO3��HClO4���ɷֱ��ʾΪ(HO)ClO2��(HO)ClO3��HClO3��ClΪ+5�ۣ�HClO4��ClΪ+7�ۡ����������Ը��ߣ�����H��O֮��ĵ��Ӷ���Oƫ�ƣ��������H+����HClO4�з��ǻ�������HClO3�࣬��HClO4���Ա�HClO3ǿ����
��3����A����NaCl��ˮ��Һ�У�NaCl����������ƶ��������Ӻ������ӣ����Ӽ����ƻ���A���������⣻
B��ˮ�����д��ڼ��Թ��ۼ���B�������⣻
C���������ṩ�չ����ˮ�����е���ԭ���ṩ�µ��Ӷԣ�������λ����C��ȷ��
D��ˮ���Ӽ���������D��ȷ��
��ѡBCD��
�ڸ��ݼ۲���ӶԻ������ۣ�ClO3-�ļ۲���Ӷ�����4������ԭ���ϵŵ��Ӷ�����1����ClO3-�Ŀռ乹��Ϊ�����Σ�ԭ�Ӹ�����ȣ��۵���������ͬ�ķ��ӻ����ӻ�Ϊ�ȵ����壬����ClO3-�ĵȵ������������SO32-��IO3-��BrO3-��
��4�������У���Na+Ϊ��������������ǰ��������6��Cl-��Na+λ��Cl-��Χ�ɵİ���������ģ���ͼ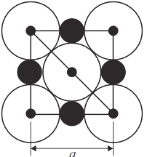 ���ö�����ı߳�=ͼ�жԽ��߳���һ��=
���ö�����ı߳�=ͼ�жԽ��߳���һ��=![]() ��
��
��5������ͼ ��NaCl�������������ӵ���̾���Ϊanm��һ�뼴
��NaCl�������������ӵ���̾���Ϊanm��һ�뼴![]() nm��Cl-�뾶Ϊ�Խ��ߵ�
nm��Cl-�뾶Ϊ�Խ��ߵ�![]() ����Ϊ
����Ϊ![]() nm����ͼ
nm����ͼ 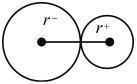 ��Na+�뾶Ϊ(
��Na+�뾶Ϊ(![]() nm-
nm-![]() nm)������Na+�뾶��Cl-�뾶֮��Ϊ(
nm)������Na+�뾶��Cl-�뾶֮��Ϊ(![]() nm-
nm-![]() nm)��
nm)��![]() nm=0.414 ��
nm=0.414 ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£���1 mol A(g)��1 mol B(g)����2 L�ܱ������з�����ӦA(g)+B(g)xC(g)+D(s)����t1ʱ�ﵽƽ�⡣��t2��t3ʱ�̷ֱ�ı䷴Ӧ��һ�����������������C(g)��Ũ����ʱ��仯��ͼ��ʾ�������й�˵����ȷ����

A. ��Ӧ����ʽ��x��2
B. t2ʱ�̸ı��������ʹ�ô���
C. t3ʱ�̸ı����������ȥ����D
D. t1��t3��÷�Ӧ��ƽ�ⳣ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ʹ�õĽ���֮һ�����������仯�����֪ʶ������������⡣
��1�����ˮ����εμ�1 mol/L FeCl3��Һ����Һ������ĺ��ɫ���÷�ɢϵ������ֱ���ķ�Χ��____________nm��
��2�����ӹ�ҵ��Ҫ��30 %��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭƬ����ӡˢ��·�壬��д��FeCl3��Һ��ͭ��Ӧ�����ӷ���ʽ______________________________________��ijͬѧ��FeCl3��ʴͭ��������Һ����ɽ��вⶨ��ȡ����������Һ������KSCN��Һ�ʺ�ɫ������Һ������������������________________��
��3����Ҫ��֤����Һ�к���Fe2������ȷ��ʵ�鷽����________������ĸ�������
A�����Թ��м�����Һ������KSCN��Һ������Ѫ��ɫ��֤������Fe2����
B�����Թ��м�����Һ���������Ը��������Һ������ɫ��֤������Fe2����
C�����Թ��м�����Һ���ȵ���KSCN��Һ���������ٵμ���ˮ������Ѫ��ɫ��֤��ԭ��Һ�к���Fe2��
��4�����ӷ�Һ�л���ͭ�������»��FeCl3��Һ���ʵ�鷽�����£�
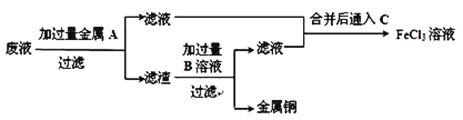
��д������ʵ�����й����ʵĻ�ѧʽ��A��__________��B��__________��
��д��ͨ�� C�Ļ�ѧ����ʽ_____________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������Ⱦ���������������Ļ�ѧҩ��ȴ����ӭ��������ij�������佫��SO2��NO������O3Ԥ��������CaSO3ˮ����Һ���ա�O3����������SO2��NOx����Ҫ��Ӧ���Ȼ�ѧ����ʽΪ
NO(g)+O3(g)=NO2(g)+O2(g) ��H=-200.9 kJ��mol-1
NO(g)+1/2O2(g)=NO2(g) ��H=-58.2 kJ��mol-1
SO2(g)+O3(g)=SO3(g)+O2(g) ��H=-241.6kJ��mol-1
(1)ʵ��������ڳ����·�Ӧ��3NO(g)+O3(g)=3NO2(g)�����Է�����˵��ԭ��_____��
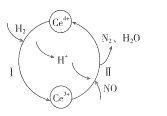
(2)��NO��H2�Ļ������ͨ��Ce(SO4)2��Ce2(SO4)3�Ļ����Һ�У���ת��������ͼ��ʾ����д����ӦI�����ӷ�Ӧ����ʽ��___________����ӦII�����ӷ���ʽ��________________��
(3)��CaSO3ˮ����Һ����������������������Һ�м���Na2SO4��Һ�������NO2���������ʣ�����Ҫԭ����___________���ﵽƽ�����Һ��c(SO32-)=__________����c(SO42-��Ksp(CaSO3)��Ksp(CaSO4)��ʾ�ݡ�
(4)���������ﻹ����ͨ����ⷨ������õ����������������Ϊ�����������մɣ���ͼд�������ĵ缫��Ӧ����ʽ��_______________��
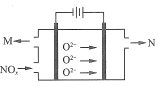
(5)��֪ SeO2��SO2�Ļ��������ˮ���տ��Ƶõ����������˵ô��������IJ��������������±���ʾ��ij���ղ����������ķ����ᴿ��ô�����������������Ŀ����_____��

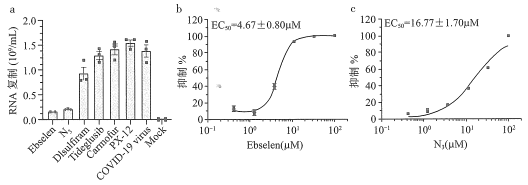
(6)����ҹ�ѧ������COVID-19���°е�Mpro����ø��ͨ���Ƚ�ɸѡ�ֶΣ���FDA���������к��ٴ�ʵ��ҩ���У�������ҩEbselen(![]() )��ϸ��ʵ����չ�ֳ�����Ŀ�����Ч�������ڴ�ǰ�о�����ƵĶ��ֹ�״����Mpro����ø�����Ƽ�N3,������ҩ�ﶼ�����Ʋ����ĺ�����ĸ��ơ�Ebselen���Ʋ������Ƶ����Ũ��Ϊ_____________
)��ϸ��ʵ����չ�ֳ�����Ŀ�����Ч�������ڴ�ǰ�о�����ƵĶ��ֹ�״����Mpro����ø�����Ƽ�N3,������ҩ�ﶼ�����Ʋ����ĺ�����ĸ��ơ�Ebselen���Ʋ������Ƶ����Ũ��Ϊ_____________![]() ,�Ƚ�N3������ЧŨ���������ױȵ����ơ���Ӱ�컯ѧ��Ӧ���ʵ����ط���������ҩ�������ԭ��Ϊ_____________��
,�Ƚ�N3������ЧŨ���������ױȵ����ơ���Ӱ�컯ѧ��Ӧ���ʵ����ط���������ҩ�������ԭ��Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������,���ڵ���ʵ���__________(�����,��ͬ),���ڷǵ���ʵ���________,����ǿ����ʵ���__________,����������ʵ���________��
��CH3CH2OH ��CH3COOH ������̬KNO3 ��SO3 ������ ��HClO ��NaHCO3 ����ˮ ��Cl2 ��BaSO4 Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��������ˮ����A����������������Ӱ������ҩ�ȡ������ǶԸû�������Ʊ���������ʵ�鷽����

��ش��������⡣
(1)�����������Ʊ����ռ���������������2.00g���ۣ�������FeS���������������ʣ�����150mL��ƿ�У�����25mL 3 mol/L H2SO4,ˮԡ���ȡ���Ӧ��ϳ��ȹ��ˣ���Ӧװ������ͼ��ʾ��ÿ��װ������һ�Σ���
��ʹ������װ�����ʵ�飬ָ��װ������˳��a______________��
�ڷ�Ӧ��Ϻ���ȹ��˵�Ŀ����________________________��
(2)��������ˮ����A���Ʊ�������Һת����������ʢ��50 mL 1 mol/L H2C2O4��Һ��250mL�ձ��У������¼��������ڣ�һ��ʱ���õ�����ɫ����������Ҫ�ɷ�ΪA)��
����֪A��������������Ϊ31%,�仯ѧʽΪ____________��
��3.6g A �����������¼��ȣ����յõ�1.44g ���廯�����д���ù����з�����Ӧ�Ļ�ѧ����ʽ��___________________________��
������ʵ�鷽���е�����������������������Եõ����߲��ʵ�A,���û�ѧƽ������֪ʶ��������ָ���õ��������ӻ��Ǽ������������________��
(3)��������ˮ����A���ȵIJⶨ����ȡmg������100mL�ձ��У���2 mol/L H2SO4�ܽ⣬ת����250 mL����ƿ�в���2 mol/L H2SO4���ݡ���ȡ25.00mL��Һ�� 250 mL ��ƿ�У��Ⱥ���Ũ��Ϊc mol/L�ı����������Һ�ζ���ƽ�вⶨ���Σ�ƽ�����ĵζ���VmL(�������ʲ�����ζ���Ӧ����
��д���ζ������з�����Ӧ�����ӷ���ʽ��___________________________ ��
���г���ʾ������A�Ĵ��ȵļ���ʽ��_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��д����ʽ
��1������������ˮ�����ӷ���ʽ_________________��
��2�������������ڿ����б������Ļ�ѧ����ʽ___________��
��3��������ʴ�����Ļ�ѧ����ʽ____________________��
��4����ȥNa2CO3�����л��е�NaHCO3�Ļ�ѧ����ʽ____________��
��5����ȥFeCl2��Һ�л��е�FeCl3�����ӷ���ʽ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȼú�����к���һ�����Ķ���������ɴ�����Ⱦ��һ�������£�ͨ�����з�Ӧ����ʵ��ȼú��������Ļ��գ�2CO��g��+SO2��g��![]() 2CO2��g��+S��l�� H�� ��ش�
2CO2��g��+S��l�� H�� ��ش�
����֪CO��g��+![]() O2��g��=CO2��g�� ��H1=-a kJ��mol-1
O2��g��=CO2��g�� ��H1=-a kJ��mol-1
S��s��+O2��g��=SO2��g�� H2=-b kJ��mol-1
S��l��=S��s�� H3=-c kJ��mol-1
����H=_______kJ��mol-1��
��һ���¶��£���2L�����ܱ�������ͨ��2molCO��1molSO2���ڲ�ͬ�����½��з�Ӧ��2CO��g��+SO2��g��![]() 2CO2��g��+S��l������Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
2CO2��g��+S��l������Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
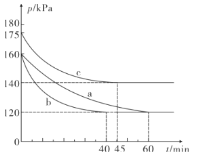
��ͼ������ʵ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v��CO���ɴ�С�Ĵ���Ϊ___�� ��ʵ����ţ��� ��a����ȣ�c��ı��ʵ������������___��
����b�鷴Ӧ���е�40minʱ�ﵽƽ��״̬����ʱ���������������ܶȱȷ�Ӧǰ������12.8 g��L-1����CO�����ʵ���Ũ��c��CO��=___����Ӧ�ڴ������µĻ�ѧƽ�ⳣ��K=___
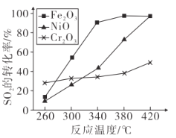
������������ͬ��������ͬʱ��SO����ת�����淴Ӧ�¶ȵı仯����ͼ��260��Cʱ��______����Fe2O3��NiO��Cr2O3����������Ӧ������죻Fe2O3��NiO��������ʹSO2��ת���ʴﵽ��ߣ������Ǽ۸����أ�ѡ��Fe2O3����Ҫ�ŵ���_______��
��SO2��O2���ӽ���Ĥȼ�ϵ��ʵ���������ᡢ���硢��������Ŀ�꣬�˵�صĸ�����ӦʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�ý���ʵ�飬��Һ��A��μ��뵽����B�У�������������ȷ���ǣ�������
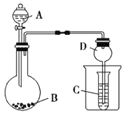
A.��AΪŨ���ᣬBΪKMnO4��C��ʢƷ����Һ����C����Һ��ɫ
B.��AΪ���ᣬBΪ���ǣ�C��ʢNa2SiO3����C����Һ�б����
C.��AΪŨ��ˮ��BΪ��ʯ�ң�C��ʢAlCl3��Һ����C���Ȳ���������������ܽ�
D.ʵ������D������ֹ��Һ����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com