
 2CuSO4+2H2O ) ���������������һ������
2CuSO4+2H2O ) ���������������һ������
 2CuO Fe+CuSO4��FeSO4+Cu����ÿ��l�֣���2�֣�
2CuO Fe+CuSO4��FeSO4+Cu����ÿ��l�֣���2�֣� 2CuO��Fe+CuSO4��FeSO4+Cu����
2CuO��Fe+CuSO4��FeSO4+Cu����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����ȡ��Һ�� | B�����ȷֽ⣻ | C�������ᾧ�� | D����Һ��E������F�����˵ȣ��뽫�ᴿ����뷽����������ں�������ϡ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��ij��Һ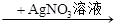 ���ɰ�ɫ������˵��ԭ��Һ����Cl�� ���ɰ�ɫ������˵��ԭ��Һ����Cl�� |
B��ij��Һ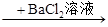 ���ɰ�ɫ������˵��ԭ��Һ����SO42�� ���ɰ�ɫ������˵��ԭ��Һ����SO42�� |
C��ij��Һ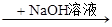 ���ɺ��ɫ������˵��ԭ��Һ����Fe3+ ���ɺ��ɫ������˵��ԭ��Һ����Fe3+ |
D��ij��Һ ������ɫ���壬˵��ԭ��Һ����CO32�� ������ɫ���壬˵��ԭ��Һ����CO32�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ͼ�У�����ͷ�ι��е�ˮ���뵽�������У��ܿ���U������īˮ���� |
| B����ͼ�У��۲쵽ʪ�����ɫ��������ɫ����β��ȫ���������պ�ϡ��������ձ��У�����Һ�����ԣ����ܿ����л���ɫ�������� |
| C����ͼ�У��۲쵽�����ڻ���ɫ����ʧ����Ͳ�ڱ�����״�������ɡ������������ڼ����������ڹ��������·����˼ӳɷ�Ӧ |
| D����ͼ�У����ԱȽ�KMnO4��Cl2��S��ԭ�Ե����ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���٢� | C���ڢ� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
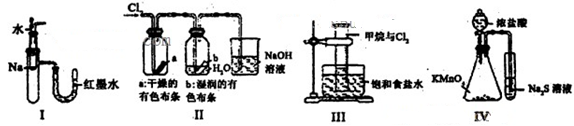
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
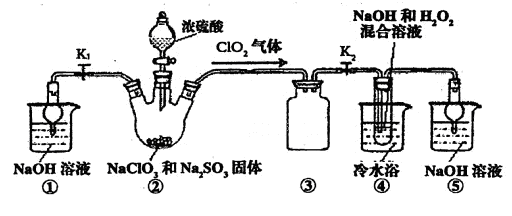
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
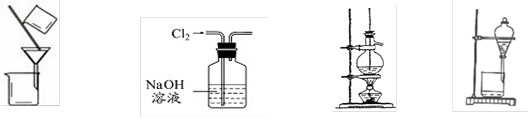
| A������NaCl��Һ��CaCO3 | B����ȥCl2�к��е�HCl | C��ʯ�ͷ��� | D�������Ҵ���ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥ�Ҵ��л��е������������������Һ��Һ |
| B����ȥ����CO2�е�SO2��������ͨ������̼������Һϴ�� |
| C����ȥKCl��Һ�е�K2CO3���������BaCl2��Һ����� |
| D����ȥ����CO2�е�CO��������ͨ��װ����������ͭ�ļ��ȹ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com