
Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ25��00mL����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
��1���ζ�ʱ��KMnO4��ҺӦװ�� �����ʽ�ζ��ܡ���ʽ�ζ��ܡ� ���У��ﵽ�ζ��յ������Ϊ ��
��2�����ζ�ʱ��û�ñ�Һϴ�ӵζ��ܣ���ʹ�ò�����Һ�����ʵ���Ũ��_ _ (�ƫ�ߡ���ƫ�͡�����Ӱ�족)
��3�����ζ�ʱ����Ӧǰ������ζ����ֱ�Ϊa��b����ʵ�������������Һ�����ʵ���Ũ��Ϊ mol/L��
��4���ڸ���Һ��KOH��Һ��Ӧ���õ�0��1 mol/L KHC2O4��Һ�У�c(C2O42-)��c��H2C2O4�������й�ϵ��ȷ���� ��
| A��c��K+��+c��H+��=c(HC2O4-)+c(OH-)+c(C2O42-) |
| B��c(HC2O4-)+ c (C2O42-)+ c��H2C2O4��=0��1mol/L |
| C��c(H+)��c��OH-�� |
| D��c��K+��=c��H2C2O4��+c(HC2O4-)+c(C2O42-) |
��1����ʽ�ζ��� (2��) �μ����һ��KMnO4����ƿ����Һ����ɫͻ��Ϊ�Ϻ�ɫ���Ұ��������ɫ���ָ��� (2��)
��2��ƫ�� (2��)
��3��0��1c(b-a) (3��)
��4��BD (3��)
�������������1����ΪKMnO4����ǿ�����ԣ��ḯʴ�ܣ���Ӧ����ʽ�ζ���ʢװ���ʴ�Ϊ��ʽ�ζ��ܡ���KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KnO4��Һʱ����Һ������ɫ��Ϊ��ɫ���ʴ�Ϊ�����������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ㣻��2�����ζ�ʱ��û�ñ�Һϴ�ӵζ��ܣ��൱�ڰѱ���Һ������ϡ�ͣ��������ĵĵı�Һ���ƫ�ߣ���ʹ�ò�����Һ�����ʵ���Ũ��ƫ�ߡ���3�����ζ�ʱ����Ӧǰ������ζ����ֱ�Ϊa��b�����Һ�����Ϊ(b-a)ml����ñ�ҺKMnO4�����ʵ���Ϊ��c(b-a)/1000mol������2KMnO4-----5H2C2O4��ϵʽ��������������Һ�����ʵ���Ũ��Ϊ0��1c(b-a)mol/L����4����c(C2O42-)��c��H2C2O4����˵��HC2O4-����̶ȴ���ˮ��̶ȡ� A�� c��K+��+c��H+��=c(HC2O4-)+c(OH-)+c(C2O42-)����غ��ϵʽ��д���� B��c (HC2O4-)+ c (C2O42-)+ c��H2C2O4��=0��1mol/L�������غ㣬��ȷ�� HC2O4-����̶ȴ���ˮ��̶ȣ��� C�� c(H+)��c��OH-������ D��c��K+��=c��H2C2O4��+c(HC2O4-)+c(C2O42-)���غ����ʽ����ȷ��
���㣺���⿼�����к͵ζ�ʵ�飬�Ѷ����У�ע�����ղ��Ậ���ļ��㷽�����к͵ζ��е����������ɽ��


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����ͼ�Ǽ���ȼ�ϵ��ԭ��ʾ��ͼ���ش��������⣺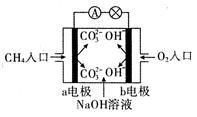
�ٵ�صĸ����� ���a����b�� �������ü��ĵ缫��Ӧʽ�� ��
�� ��ع���һ��ʱ���������Һ��pH �����������С�����䡱����
��2�����ü���ȼ�ϵ�ؼ���ͼ��ʾ��װ����ɵ�ⱥ��ʳ��ˮ��ʵ�飬Ҫ��ⶨ����������������������������������ԡ�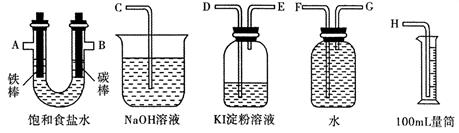
���������ʵ��װ��ʱ�����ӿڵ���ȷ����˳��Ϊ�� �� �� �� A �� B �� �� �� ��
��ʵ���У���ʢ�� KI ������Һ�������з�����Ӧ�����ӷ���ʽΪ ��
����֪����ʳ��ˮ50mL��ijʱ�̲�� H2���Ϊ56mL ����״��������ʱ��Һ pH ԼΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��25��ʱ��pH=12.0��KOH��Һ�У���ˮ�������C��OH-��=_______mol/L��pH=12.0��K2CO3��Һ�У���ˮ���������C��OH-��=_______mol/L��
��2��������0.1mol��L-1 CH3COONa��ҺPH=8
�������ӷ���ʽ��ʾCH3COONa��ҺPH=8��ԭ��:
�ڰ����ʵ���Ũ���ɴ�С˳�����и���Һ�и�������ˮ���ӳ��⣩
��3��FeCl3��ˮ��Һ�� ����ᡱ�����С���������ԣ�ԭ���ǣ������ӷ���ʽ��ʾ���� �� ʵ����������FeCl3����Һʱ������FeCl 3���������� �У�Ȼ����������ˮϡ�͵������Ũ�ȣ��� ����ٽ����������ơ�����ˮ�⣬����õ����ǻ��ǵ���Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����л�Ϻ���Һ��pH����lg2=0.3 lg5=0.7��
��1����pH=2��pH=4������ǿ����Һ�������ϣ���pH=_______��
��2����pH=12��pH=14������ǿ����Һ�������ϣ���pH=_________��
��3����pH=2��H2SO4��Һ��pH=10��NaOH��Һ�������ϣ���pH=_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪ij��Һ��ֻ����OH����H+��Na+��CH3COO���������ӣ�ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
| A��c (CH3COO��)��c (Na+)��c (H+)��c (OH��) | B��c (CH3COO��)��c (Na +)��c (OH��)��c (H+) |
| C��c (CH3COO��)��c (H+)��c (Na+)��c (OH��) | D��c (Na+)��c(CH3COO��)��c (OH��)��c (H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʢ�Cu����Һ̬SO2����CH3COOH����NaHCO3����Ba(OH)2��Һ��������NaCl����ϡ��ˮ����BaSO4����H2O
��1������ǿ����ʵ��� ������ţ� ����������ʵ��� ������ţ�
��2�������£�0.1 mol��L-1NaHCO3��Һ��pH����8������Һ��Na����HCO3�D��CO32�D��OH�D��������Ũ���ɴ�С��˳��Ϊ�� ��NaHCO3ˮ������ӷ���ʽ ��
��3��Ba(OH)2��һ��ǿ����ʣ�����25�桢pH��13��Ba(OH)2��Һ��
�ٸ�Ba(OH)2��Һ�����ʵ���Ũ��Ϊ___________________��
����ijŨ��������Һ������ȣ�������֮�ȣ�1 ��9��Ϻ�������ҺpH��11����������Һ��������ڻ��ǰ����Һ������ͣ�����������Һ��pH��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ����ģ�����ij�Ͼɺ�����������Ҫ�ɷ�ΪNiO������Fe2O3��CaO��CuO��BaO�ȣ�����Ni2O3���乤������Ϊ��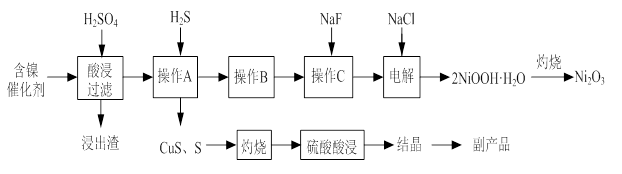
ͼ�� ͼ��
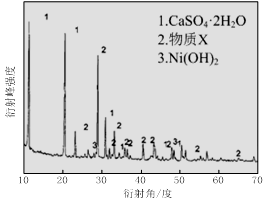
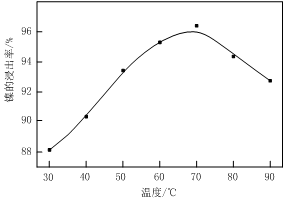
��1������ͼ����ʾ��X��������ͼ�ף���֪����������������Ҫ�ɷ֣����С�����X��Ϊ ��ͼ���ʾ���Ľ��������¶ȵĹ�ϵ���������¶ȸ���70��ʱ�����Ľ����ʽ��ͣ���������Ni(OH)2����������ԭ���� ��
��2�����������С�����Ʒ���Ļ�ѧʽΪ ��
��3����֪�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
| ��ʼ������pH | 1.5 | 6.5 | 7.7 |
| ������ȫ��pH | 3.7 | 9.7 | 9.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������ѧ��ѧ�г������ᣬ��һ�������£�CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH CH3COO-+H+ ��H��0��
CH3COO-+H+ ��H��0��
��1�������£��� pH =5��ϡ������Һ�У�c(CH3COO-)��____________(��ʽ�����ػ���)�����з����У�����ʹ0��10 mol��L-1 CH3COOH�ĵ���̶��������______?
a����������0��10 mol��L-1��ϡ���� b������CH3COOH��Һ
c����ˮϡ����0��010 mol��L-1 d����������������
e�����������Ȼ��ƹ��� f����������0��10 mol��L-1��NaOH��Һ
��2������������пͶ��������pH������3�Ĵ����������Һ�У�������ַ�Ӧ����ֻ��һ����Һ����п��ʣ�࣬�����������������V(����)_________V(����)����Ӧ���������Ϊ��v(����)_________v(����)��(��д������������������)
��3��ijͬѧ��0��1000mol/LNaOH��Һ�ֱ�ζ�20��00mL 0��1000mol/LHCl��20��00mL0��1000mol/L CH3COOH���õ���ͼ��ʾ�����ζ����ߣ�������й����⣺
��NaOH��Һ�ζ�CH3COOH��Һ�������� ���ͼ1����ͼ2������
��a�� mL��
��4�������£���0��1 mol/L�����0��1 mol/L��������Һ��ϣ�������ҺΪ���ԣ�������Һ�и����ӵ�Ũ�Ȱ��ɴ�С����Ϊ_______________________________��
��5����֪��90��ʱ��ˮ�����ӻ�����ΪKw = 3��80��10-13���ڴ��¶��£���pH=3�������
pH = 11������������Һ�������ϣ�������Һ�е�c(H+)=____________(������λ��Ч����)mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij�¶��£���ˮ�������c(H��)=2��10��7mol/L��
��1�����¶��£�0.1mol/L �����PH= ��0.1mol/L NaOH��Һ�е�c(H��)=
��2�����¶��£�pH=13��NaOH��Һ�е�c(OH��)��pH=11��NaOH��Һ�е�c (OH��)�� �������������Ե������ϣ�������ҺpHֵΪ ����lg2=0.3��lg3=0.5��lg5=0.7��
��3�����¶��£������ʵ���Ũ�ȵ�NaOH��Һ�����ᰴ3��2����Ȼ�ϣ������ǻ��ʱ������仯����������Һ��pHֵΪ12�����ǵ�Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com