
����Ŀ���Ժ��ܷϴ���(��Ҫ�ɷ�ΪCo��Fe��SiO2)Ϊԭ����ȡ���������ܵ�����������
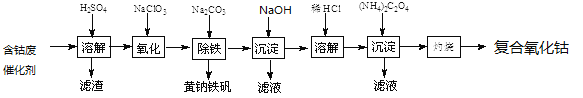
��1����H2SO4�ܽ��������õ���������_________(�ѧʽ)��������ϴ��2��3�����ٽ�ϴҺ����Һ�ϲ���Ŀ����____________________��
��2���ڼ��Ƚ��������¼���NaClO3����Fe2+������Fe3+����Ӧ�����ӷ���ʽ��___________________��
��3����֪�����軯�صĻ�ѧʽΪK3[Fe(CN)6]�������軯�صĻ�ѧʽΪK4[Fe(CN)6]��
3Fe2++2[Fe(CN)6]3-=Fe3[Fe(CN)6]2��(��ɫ����)
4Fe3++3[Fe(CN)6]4-=Fe4[Fe(CN)6]3��(��ɫ����)
ȷ��Fe2+�Ƿ�������ȫ�ķ�����__________________��(����ѡ����Լ������軯����Һ�������軯����Һ���ۡ�KSCN��Һ)
��4�������������Һ�м���������Na2CO3������ȣ�ʹ֮���ɻ�������[Na2Fe6(SO4)4(OH)12]������д���÷�Ӧ�����ӷ���ʽ��_______________________��
��5�����������ĵ���ƽ�ⳣ���ĸ���������pK��ʾ�������±��������ж�(NH4)2C2O4��Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ__________________��
H2C2O4 | pKa1= l.25��pKa2=4.13 |
NH3��H2O | pKb=4.76 |
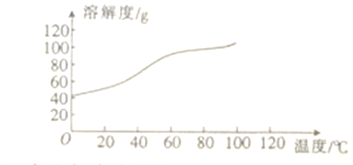
��6����֪CoCl2���ܽ��������ͼ��ʾ�����ʽ̼�����м�������ϡ������ȱ߽�������ȫ�ܽ������ȹ�����ԭ����_____________________��
��7��ȷ��ȡ1.470gCoC2O4���ڿ����г�����յ�0.814g���������ܣ�д�����������ܵĻ�ѧʽ��_________________________��
���𰸡� SiO2 �����Ԫ�ص������� 6Fe2++6H++ClO3-![]() 6Fe3++Cl-+3H2O ȡ�������������Һ���Թ��еμӼ������軯����Һ��������ɫ�������ɣ���˵��Fe2+��ȫ�������� 6Fe3++4SO42-+6H2O+2Na++6CO32-=Na2Fe6(SO4)4(OH)12��+6CO2�� c(NH4+)>c(C2O42-)>c(H+)>c(HC2O4-)>c(OH-) ��ֹ���¶Ƚ�����CoCl2�������� Co5O7
6Fe3++Cl-+3H2O ȡ�������������Һ���Թ��еμӼ������軯����Һ��������ɫ�������ɣ���˵��Fe2+��ȫ�������� 6Fe3++4SO42-+6H2O+2Na++6CO32-=Na2Fe6(SO4)4(OH)12��+6CO2�� c(NH4+)>c(C2O42-)>c(H+)>c(HC2O4-)>c(OH-) ��ֹ���¶Ƚ�����CoCl2�������� Co5O7
����������1���ܷϴ�������ϡ���ᣬ����Co+H2SO4=CoSO4+H2����Fe+H2SO4=FeSO4+H2�������������Dz��ܵĶ���������ϴҺ����Һ�ϲ������ϴ�Ӻ���Һ���ܵ������ʣ��ʴ�Ϊ��SiO2������ܵ�Ԫ�ص������ʣ�
��2���������ӱ���������������������ӣ�1mol����������ʧȥ1mol�ĵ��ӣ���1mol����������ӵõ�6mol�ĵ��ӣ����ݵ��ӵ�ʧ�غ㣬��֪���ӷ���ʽΪ��6Fe2++6H++ClO3-![]() 6Fe3++Cl-+3H2O��
6Fe3++Cl-+3H2O��
��3��ȡ���������Һ�������Թ��У��μӼ������軯����Һ��������ɫ�������ɣ���Fe2+��ȫ����������
��4��������������̼���Ʒ���˫ˮ��õ��������������ӷ���ʽΪ��6Fe3++4SO42-+6H2O+2Na++6CO32-=Na2Fe6(SO4)4(OH)12��+6CO2����
��5������pK�Ĵ�С��ϵ�������жϳ����볣���Ĵ�С��ϵΪKa1> Ka2>Kb�����Զ�Ӧ���ӵ�ˮ��̶ȴ�СΪNH4+>C2O42->HC2O4-��ˮ��̶�Խ����Һ��ʣ������Ũ�Ⱦ�ԽС��������Һ�е�����Ũ�ȴ�С˳��Ϊ��c(NH4+)>c(C2O42-)>c(H+)>c(HC2O4-)>c(OH-)
��6��CoCl2���ܽ�����߿�֪�����¶ȵ����ߣ�CoCl2���ܽ���������Գ��ȹ��ˣ���ֹ�¶Ƚ����Ȼ����������ʴ�Ϊ����ֹ���¶Ƚ��ͣ�CoCl2����������
��7��CoC2O4������Ϊ1.470g�������ʵ���Ϊ0.01mol��CoԪ������Ϊ0.59g��������������Ϊ0.814g������������Ԫ������Ϊ0.814g-0.59g=0.224g������������Coԭ����Oԭ�����ʵ���֮��Ϊ0.01mol��![]() ��5��7����Co������ΪCo5O7���ʴ�Ϊ��Co5O7��
��5��7����Co������ΪCo5O7���ʴ�Ϊ��Co5O7��


 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������ϩ����֤�����ʣ���ش��������⣺
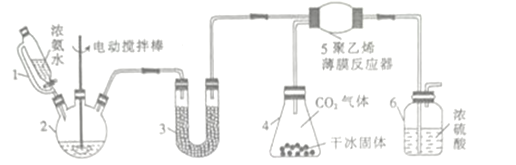
��1��д�����Ҵ�Ϊԭ����ȡ��ϩ�Ļ�ѧ����ʽ��________________________________________��
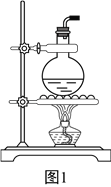
��2��ijͬѧ��ʹ����ͼ1��ʾװ����ȡ��ϩ������������еĴ���________________________��
ʵ������з�����ƿ�г��ֺ�ɫ���壬��ᵼ�����ɵ���ϩ�������������壬��д��������������Ļ�ѧ����ʽ��__________________________________________________��
��3��Ҫ��֤��ϩ�Ļ�ѧ���ʣ�װ����ͼ2��ʾ��β������װ������ȥ�����뽫���߿��е�װ�ò�����������������Լ���______________
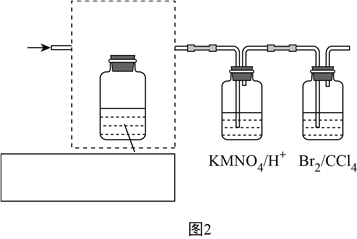
��4����Щͬѧ�����������Ϊԭ����ȡ��ϩ���÷�Ӧ�Ļ�ѧ����ʽΪ��____________________������������Ϊԭ�ϣ�ͼ2�����߿��ڵ�װ�ã���ܡ����ܡ���__________ʡ�ԣ���˵�����ɣ�____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A.���ά����Ҫ�ɷ���SiO2
B.��ҵŨ����ͨ���ʻ�ɫ
C.ʵ���ҿ�����NaOH��Һ����SO2��NO2
D.Cl2���к�ǿ�������ԣ��ڻ�ѧ��Ӧ��ֻ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(H2NCOONH4)��һ���ֽ⡢��ˮ��İ�ɫ������ij�о�С�����������ƹ��塢Ũ��ˮ���ɱ���Ϊԭ���Ʊ���������淋�ʵ��װ����ͼ��ʾ������Ҫ��Ӧ��ԭ��Ϊ2NH3(g)+CO2(g)![]() NH2COONH4(s) ��H<O��
NH2COONH4(s) ��H<O��
��1������2��������________������3��ʢװ�Ĺ�����_________����������______________��
��2������6��һ�������ǿ���ԭ��������Ӧ����ϵ����ַ�Ӧ������Ӧ���ڹ۲쵽װ����Ũ�����в������ݣ���Ӧ��______________(�����ӿ����� �������������ı���)�������������ʡ�
��3����һ���Ʊ��������ᰱ�ķ�Ӧװ��(Һ��ʯ����CCl4���䵱���Խ���)��ͼ��ʾ��
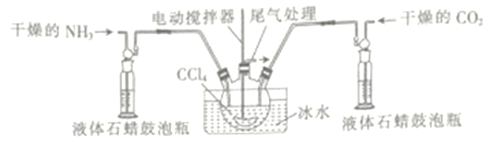
��Һ��ʯ������ƿ��������_____________________ ��
�����ޱ�ˮ�����������ֽ���������[CO(NH)2]2��д��������������ȷֽ�Ļ�ѧ����ʽ��_______________________��
�۵�CCl4Һ���в����϶ྦྷ��������ʱ������ֹͣ��Ӧ�����˷���õ��ֲ�Ʒ��Ϊ�˽����ôֲ�Ʒ�������ɲ�ȡ�ķ�����_______________(����ĸ)��
A.���� B.����Ⱥ�� C.��ѹ���Ⱥ��
��4���Ƶõİ���������п��ܺ���̼�������̼����е�һ�ֻ���������(�����ǰ����������ˮ�ķ�Ӧ)��
����Ʒ������гɷ�̽��������д���пո�
��ѡ�Լ�������ˮ��ϡ������BaCl2��Һ������ʯ��ˮ��AgNO3��Һ��ϡ���ᡣ
ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ����������Ʒ���Թ�������������ˮ�������ܽ� | �õ���ɫ��Һ |
����2�����Թ��м��������BaCl2��Һ������ | ����Һ������ǣ���֤�������в���̼��� |
����3�����Թ��м�������___________ | _____________________________�� ��֤�������к���̼����� |
�ڸ��ݢٵĽ�����ȡ15.8g�������ᰱ��Ʒ������������������Һ��ִ�����������ϴ�ӡ������ó�������Ϊ1.97g������Ʒ�а�������淋���������Ϊ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ģ�������������������ͭ�������������ϡ���Դ��ҵ�����ȡ�
��1��������Һ��Na2SO3��H2SeO2��H2SeO4��ԭΪ�����ʵķ�Ӧ������
H2SeO3(aq)+2SO2(g)+H2O(l)=Se(s)+2H2SO4(aq) ��H1
2H2SeO4(aq)+Se(s)+H2O(l)=3H2SeO3(aq) ��H2
H2SeO4(aq)+3SO2(g)+2H2O(l)=Se(s)+3H2SO4(aq) ��H3
����H2=_____________(����H1����H3��ʾ)��
��2��H2S��CO2������Cu2O�������·�Ӧ�������ʻ���(COS)���ʻ���ĽṹʽΪ____________________��
��3����ͭ����������Ƭ�����������һ��Ũ�ȵ�NaCl��NaOH�Ļ����Һ�ɵõ�Cu2O����������Һ�����ʵ��й�ת����ͼ1��ʾ��
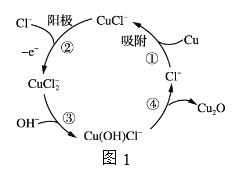
�������ĵ缫��ӦʽΪ_________________________��
�ڵ��һ��ʱ���������Һ�в���һ������_________________�ɽ���Һ�ָ�����ʼ״̬��
��4��������������Cu2O���������ڹ�ҵ�Ϻϳɼ״���
CO(g)+2H2(g) ![]() CH3OH(g) ��H=akJ��mol-1��
CH3OH(g) ��H=akJ��mol-1��
��![]() =1��Ͷ�ϱȽ�H2��CO����VL�ĺ������������У���һ�������·�����Ӧ�����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ2��ʾ��
=1��Ͷ�ϱȽ�H2��CO����VL�ĺ������������У���һ�������·�����Ӧ�����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ2��ʾ��
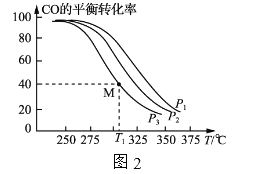
��a_____(����>������<��)0��ѹǿp1��p2��p3��С�����˳����___________________��
��T1��ʱ����������г���2.0molH2��2.0molCO����������Ӧ��5min��Ӧ�ﵽƽ��(M��)����0��5min�ڣ�v(H2)=____mol��L-1��min-1��M���Ӧ�����·�Ӧ��ƽ�ⳣ��
Ϊ________________��
��5����CuClˮ�����ȷֽ�ɵõ�����Cu2O��CuClˮ��ķ�ӦΪCuC(s) +H2O(l) ![]() CuOH(s)+Cl-(aq)+H+(aq)���÷�Ӧ��ƽ�ⳣ��K����¶���Kw��Ksp(CuOH)��Ksp(CuCl)�Ĺ�ϵΪK=________________��
CuOH(s)+Cl-(aq)+H+(aq)���÷�Ӧ��ƽ�ⳣ��K����¶���Kw��Ksp(CuOH)��Ksp(CuCl)�Ĺ�ϵΪK=________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ɳ�Ѫ��ƽ����������Ѫ�ܵĹ��ܣ���ṹ��ʽ����ͼ�����жԸ����ʵ������У���ȷ����
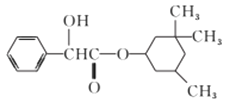
A. ���л��ﱽ���ϵĶ��ȴ��ﹲ��8��
B. ���л��������������7��̼ԭ�ӹ�ƽ��
C. ���л��������Br2��CCl4��Һ�����ӳɷ�Ӧʹ֮��ɫ
D. 1mol���л���������2molNaOH������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������������̬������ʵ��������Ȼ�ĺ�г������
������������Ӿ�����ЧӦ����__________������ĸ����
a.ֲ������ b.ȼú��ů c.��������
�����з��Ρ���ɫ��Ⱦ������ȷ������_____________������ĸ����
a.ʹ�ÿɽ������� b.¶����շϾ����� c.ֱ������Ͼ�����
��Ϊ���������Ⱦ����������ѽ�ֹȼ���̻�������ֹȼ���̻����ı�ʶ��_____������ĸ����

��2������ʹ�û�ѧ֪ʶ��������ǵ�����������
ijƷ������ijɷ��и��͡�ɽ����ء������Ƶȡ�
������������ɷ��У����ڷ���������_______________��
�ڸ��͵Ľṹ��ʽΪ____________����֬ˮ������ɸ��ͺ�_____________��
�۷�����(NaF)���������е��ǻ������[Ca5(PO4)3OH]��Ӧ�����ɸ����ܵķ������[Ca5(PO4)3F]���Ӷ��ﵽ����ȣ�ݵ�Ŀ�ġ�д���÷�Ӧ�Ļ�ѧ����ʽ��____________________��
��3�����·�չ���ϼ������ƶ��������Ľ�����
��ʯīϩ������ͼ��������̫���ܵ�صĵ缫��������Ҫ������ʯīϩ��______________�ԡ�

�ڻ������̽����г��õ�ˮ�ࡢ�������ֲĵȡ�����ˮ��Ͳ������õ���ԭ����__________���ڸֲ������Ӹ�������Ԫ�ص�Ŀ����___________��
������ս������������SiC������Ϊ�������ϡ������½�̿��ʯӢ��Ӧ���Ƶ�SiC��ʯӢ�Ļ�ѧʽΪ________________�����·ֽ�Si(CH3)2Cl2Ҳ���Ƶ�SiC��ͬʱ������CH4��һ�ֳ����������壬д���÷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʷ���ķ�����ȷ���ǣ�������
A.�ӵ�ˮ����ȡ�⣺�þƾ���ȡ
B.��ȥCO������O2��ͨ�����ȵ�Cu�����ռ�����
C.��ȥKCl��Һ�е�����MgCl2����������NaOH��Һ������
D.��ȥCO2�е�����HCl��ͨ�뱥��NaHCO3��Һ���ռ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪NO��O2ת��ΪNO2�ķ�Ӧ�������£�
��2NO(g)![]() 2N2O2(g)���죩 ��H1��0 ƽ�ⳣ��K1
2N2O2(g)���죩 ��H1��0 ƽ�ⳣ��K1
��N2O2(g) +O2(g)![]() 2NO2(g) ������ ��H2��0 ƽ�ⳣ��K2
2NO2(g) ������ ��H2��0 ƽ�ⳣ��K2
����˵����ȷ����
A. ��Ӧ�����е������仯����ͼa��ʾ

B. 2NO(g) +O2(g)![]() 2NO2(g)�ġ�H=-(��H1+��H2)
2NO2(g)�ġ�H=-(��H1+��H2)
C. 2NO(g)+O2(g)![]() 2NO2(g)��ƽ�ⳣ��K=K1/K2
2NO2(g)��ƽ�ⳣ��K=K1/K2
D. ��Ӧ�ڵ����ʴ�С����2NO(g)+O2(g)![]() 2NO2(g)�ķ�Ӧ����
2NO2(g)�ķ�Ӧ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com