
����Ŀ�������£���1L0.1mol/LH2A��Һ����μ����Ũ��NaOH��Һ��������Һ�к�AԪ�ص��������ʵ�����������ҺpH�Ĺ�ϵ��ͼ������˵������ȷ���ǣ� ��
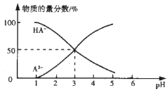
A.H2A�ĵ��뷽��ʽΪH2A=HA-+H+ HA-A2-+H+
B.�����£�Na2Aˮ��ƽ�ⳣ��Kh=10-11
C.0.1mol/LNaHA��Һ�д���c(A2-)+c(HA-)��0.1mol/L
D.�����£������ʵ���Ũ��NaHA��Na2A��Һ�������Ϻ���Һ��pH=3.0
���𰸡�B
��������
![]() ��ͼ���֪��pH=1����Һ�к���AԪ�ص���������H2A����A2-��HA-������
��ͼ���֪��pH=1����Һ�к���AԪ�ص���������H2A����A2-��HA-������![]() ��Һ��ȫ������Ϊ
��Һ��ȫ������Ϊ![]() ��˵����һ������Ϊ��ȫ���룬����
��˵����һ������Ϊ��ȫ���룬����![]() �ĵ��뷽��ʽΪ��
�ĵ��뷽��ʽΪ��![]() ��
��![]() ����A����
����A����
B��![]() ʱ
ʱ![]() ����
����![]() ˮ��ƽ�ⳣ��
ˮ��ƽ�ⳣ��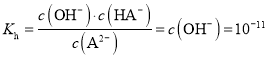 ����B��ȷ��
����B��ȷ��
C��![]() ��Һ��ȫ�����룬������
��Һ��ȫ�����룬������![]() ���ӣ����������غ��֪��
���ӣ����������غ��֪��![]() ����C����
����C����
D�������£�c��A2-��=c��HA-��ʱ��Ka=![]() =c��H+��=10-3����A2-ˮ��ƽ�ⳣ��Kh=
=c��H+��=10-3����A2-ˮ��ƽ�ⳣ��Kh=![]() =10-11��Ka��������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������Ϻ�A2-ˮ��̶�С��HA-����̶ȣ�������Һ��c��A2-����c��HA-������c��H+����10-3����Һ��pH��3����D����
=10-11��Ka��������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������Ϻ�A2-ˮ��̶�С��HA-����̶ȣ�������Һ��c��A2-����c��HA-������c��H+����10-3����Һ��pH��3����D����
��ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�������ϡ����Դ�����ϡ��Ԫ������ϵ����![]() ����
����![]() ��Ԫ�ص��ܳƣ�����λ��Ԫ�����ڱ���ͬһ���塣
��Ԫ�ص��ܳƣ�����λ��Ԫ�����ڱ���ͬһ���塣
![]() ԭ�ӵļ۲�����Ų�ʽΪ______����һ������Sc______Y
ԭ�ӵļ۲�����Ų�ʽΪ______����һ������Sc______Y![]() ����ڡ���С�ڡ�
����ڡ���С�ڡ�![]() ��
��
![]() ����������м仯��������ɷ�̼����
����������м仯��������ɷ�̼����![]()
![]() �����Ƶã�
�����Ƶã�
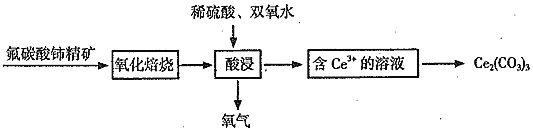
![]() �У�Ce�Ļ��ϼ�Ϊ______��
�У�Ce�Ļ��ϼ�Ϊ______��
![]() �����������ɶ�������
�����������ɶ�������![]() ���������ʱ������Ӧ�����ӷ���ʽΪ______��
���������ʱ������Ӧ�����ӷ���ʽΪ______��
![]() ���ӵ����幹�͵�����Ϊ______������ԭ�ӵ��ӻ���ʽΪ______�����ӻ������еĴ�
���ӵ����幹�͵�����Ϊ______������ԭ�ӵ��ӻ���ʽΪ______�����ӻ������еĴ�![]() �����÷���
�����÷���![]() ��ʾ������m���������γɴ�
��ʾ������m���������γɴ�![]() ����ԭ������n���������γɴ�
����ԭ������n���������γɴ�![]() ���ĵ�����
���ĵ�����![]() �籽�����еĴ�
�籽�����еĴ�![]() ���ɱ�ʾΪ
���ɱ�ʾΪ![]() ����
����![]() �еĴ�
�еĴ�![]() ��Ӧ��ʾΪ______��
��Ӧ��ʾΪ______��
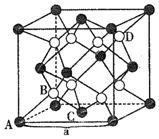
![]() ���¡���ѹ��
���¡���ѹ��![]() �������ȶ��Ļ�����㷺���ڲ�����ԭ���ܡ����ӹܵȹ�ҵ��
�������ȶ��Ļ�����㷺���ڲ�����ԭ���ܡ����ӹܵȹ�ҵ��![]() ����������өʯ�ͣ��������ӵ���λ��Ϊ______����ͼ�������������
����������өʯ�ͣ��������ӵ���λ��Ϊ______����ͼ�������������![]() 0��
0��![]() ��
��![]() ��
��![]() ������BѡΪ���������������
������BѡΪ���������������![]() 0��
0��![]() ����D���Ӵ���______λ�ã��������Ϊ______����֪�þ������ⳤ
����D���Ӵ���______λ�ã��������Ϊ______����֪�þ������ⳤ![]() �����ܶ�Ϊ______
�����ܶ�Ϊ______![]() �г�����ʽ����
�г�����ʽ����![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ�1��2-��������ķ�Ӧ�п��ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѡ�������������������Ҵ��Ʊ�1��2�����������װ������ͼ��ʾ��

�й������б����£�
�Ҵ� | 1��2-�������� | ���� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
�ܶȣ�g��cm-3 | 0.79 | 2.2 | 0.71 |
�е㣯�� | 78.5 | 132 | 34.6 |
�۵㣯�� | һl30 | 9 | -1l6 |
�ش��������⣺
��1���ڴ�ʵ���У���Ӧ����ʽΪ��_________��________��
��2��Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����_____��(����ȷѡ��ǰ����ĸ)
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��3����װ��C��Ӧ����_______����Ŀ�������շ�Ӧ�п������ɵ��������壺(����ȷѡ��ǰ����ĸ)
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4����������������δ��Ӧ��Br2�������_________ϴ�ӳ�ȥ��(����ȷѡ��ǰ����ĸ)
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��5���жϸ��Ʊ���Ӧ�Ѿ��������������_____________��
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ����________________�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����NaOH��Һ�ζ�H2C2O4��Һ����Һ����1g[c(H+)/c(H2C2O4)]�ͣ�1gc(HC2O4-)����1g[c(H+)/c(HC2O4-)]�ͣ�1gc(C2O42-)��ϵ��ͼ��ʾ������˵������ȷ����
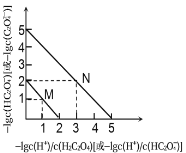
A. Ka1(H2C2O4)��������Ϊ10-2
B. ����M��ʾ��1g[c(H+)/c(H2C2O4)]�ͣ�1gc(HC2O4-)�Ĺ�ϵ
C. ��NaHC2O4��Һ�м�NaOH��c(HC2O4-)��c(C2O42-)��ȣ���ʱ��ҺpHԼΪ5
D. ��NaHC2O4��Һ��c(Na+)��c(HC2O4-)��c(H2C2O4)��c(C2O42-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ҷٷ�����ȥˮ�����л���Ⱦ���ԭ����ͼ��ʾ��������Ҷٷ�Ӧ��Fe2++H2O2== Fe3++OH-+��OH�����ɵ��ǻ����ɻ�(��OH)�ܽ����л���Ⱦ�����˵����ȷ����
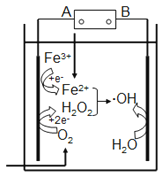
A. ��Դ��A����������B���Ǹ���
B. ������O2��Fe3+��H2O2�õ��ӷ�����ԭ��Ӧ
C. �����Ϸ����缫��Ӧ��H2O-e-==OH+H+
D. ���³�ѹ�£�����22.4LO2�����Բ���4mol��OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Һ�絼��Խ������Խǿ����������0.100 mol��L-1����ֱ�ζ�10.00 mLŨ�Ⱦ�Ϊ0.100 mol��L-1��NaOH��Һ�Ͷ��װ���(CH3)2NH����Һ�����װ���ˮ�е����백���ƣ�����Ksp[(CH3)2NH��]=l.6��10-4�������ô�������õζ���������Һ�ĵ絼����ͼ��ʾ������˵����ȷ����

A. ���ߢٴ����ζ����װ���Һ������
B. a����Һ�У�c[(CH3)2NH2+]>c[CH3]2NH��H2O]
C. d����Һ�У�c(H+)=c(OH-)+c[CH3]2NH��H2O]
D. b��c��e�������Һ�У�ˮ�ĵ���̶�������b��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Mo��������������黯��Ӧ���漰WGS ����RWGS ��Ӧ����Ҫ��Ӧʽ���£�
��1��CO��CO2���黯 CO(g)+3H2(g)=CH4(g)+H2O(g) ��H1=-206.2kJ/mol
CO2(g)+4H2(g)=CH4(g)+2H2O(g) ��H2=-165.0kJ/mol
д��CO ��ˮ������Ӧ����CO2��H2���Ȼ�ѧ����ʽ__________________________________��
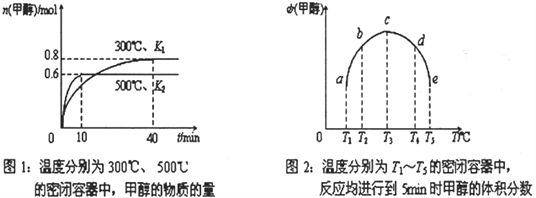
��2��CO��H2��һ�������ºϳɼ״��ķ�ӦΪ��CO(g)+2H2(g)![]() CH3OH(g) ��H�������ݻ���Ϊ1L ��a��b��c��d��e ����ܱ������зֱ����1molCO��2molH2�Ļ�����壬���£�����ʵ�飬��������������ͼ1��ͼ2��
CH3OH(g) ��H�������ݻ���Ϊ1L ��a��b��c��d��e ����ܱ������зֱ����1molCO��2molH2�Ļ�����壬���£�����ʵ�飬��������������ͼ1��ͼ2��
 ��
��
�ٸ÷�Ӧ�Ħ�H__________0 ��ѡ�<������>����=������
����500�������´�ƽ��ʱCO ��ת����Ϊ______________________��
�ۼ�����300�������´�ƽ��ʱK=________________________��
�ܽ�����d �е�ƽ��״̬ת�䵽����c�е�ƽ��״̬���ɲ�ȡ�Ĵ�ʩ��______________________��
��ij���װ����ͼ��ʾ��X��Y ��Ϊ���Ե缫��
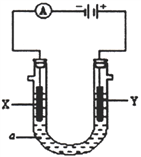
��3����a��ҺΪ���з�̪��Һ��NaNO3��Һ��ͨ��һ��ʱ���X �缫����Χ��Һ�������ǣ�________________________________��Y �缫�ĵ缫��ӦʽΪ______________________��
��4����a ��ҺΪ����CuCl2��Һ������·����0.2 mol �ĵ���ͨ��ʱ�����������缫������֮����__________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵��������ǣ� ��
A. ![]() ��Һ�У�
��Һ�У�![]()
B. ![]() ��Һ�У�
��Һ�У�![]()
C. ����![]() ��Һ��Ӧ����������
��Һ��Ӧ����������
D. ��![]() ��Һ���ɣ����յ�
��Һ���ɣ����յ�![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������ijNaOH��Һ�ֳ����ȷݣ�һ����![]() ��һԪ��HA��Һ�кͣ���������Һ�����Ϊ
��һԪ��HA��Һ�кͣ���������Һ�����Ϊ![]() ����һ����
����һ����![]() ��һԪ��HB��Һ�кͣ���������Һ���Ϊ
��һԪ��HB��Һ�кͣ���������Һ���Ϊ![]() ��������������ȷ����
��������������ȷ����![]()
![]()
A.��![]() ����˵��HA�����Ա�HB������ǿ
����˵��HA�����Ա�HB������ǿ
B.��![]() ����˵��HA�����Ա�HB��������
����˵��HA�����Ա�HB��������
C.��Ϊ��������Һ��pH��ȣ���![]() һ������
һ������![]()
D.������������Һ�������ϣ������Һ��pHһ������2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com