
����Ŀ��ú���еĵ�Ԫ����ʹ�ù����е�ת����ϵ��ͼ��ʾ��

(1)����NH3���뷴Ӧ�Ļ�ѧ����ʽΪ_______��
(2)��̿������һ�ֳ����ĺ����л������(![]() )������������ڵ�C��Nԭ����ȣ�Nԭ����������������___________(����ǿ����������)����ԭ�ӽṹ�ǶȽ���ԭ��________��
)������������ڵ�C��Nԭ����ȣ�Nԭ����������������___________(����ǿ����������)����ԭ�ӽṹ�ǶȽ���ԭ��________��
(3)��ҵ�ϳɰ����˹��̵�����Ҫ������2007�껯ѧ�Ҹ���������ض�֤ʵ�������뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ����ͼ��
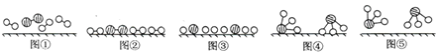
����˵����ȷ����________(ѡ����ĸ)��
a. ͼ�ٱ�ʾN2��H2�����о��ǵ���
b. ͼ����ͼ����Ҫ��������
c. �ù��̱�ʾ�˻�ѧ�仯�а����ɻ�ѧ���Ķ��Ѻ��»�ѧ��������
(4)��֪��N2(g) �� O2(g) = 2NO(g) ��H = a kJ��mol-1
N2(g) �� 3H2(g) = 2NH3(g) ��H = b kJ��mol-1
2H2(g) �� O2(g) = 2H2O(l) ��H = c kJ��mol-1
��Ӧ��ָ������³�ѹ������NH3���뷴Ӧ���Ȼ�ѧ����ʽΪ________��
(5)�ü�ӵ绯ѧ����ȥNO�Ĺ��̣���ͼ��ʾ��

����֪���ص�����������Һ��pH��4~7֮�䣬д�������ĵ缫��Ӧʽ��________��
�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ����__________��
���𰸡�4NH3+5O2 4NO+6H2O ǿ C��Nԭ����ͬһ����(����Ӳ�����ͬ)��Nԭ�Ӻ˵��������ԭ�Ӱ뾶��С��ԭ�Ӻ˶������ӵ���������ǿ bc 4NH3(g) + 6NO(g) = 5N2(g) + 6H2O(l) ��H = (3c-3a-2b) kJ��mol-1 2HSO3- + 2e- + 2H+ = S2O42- + 2H2O 2NO + 2S2O42- +2H2O = N2 + 4HSO3-
4NO+6H2O ǿ C��Nԭ����ͬһ����(����Ӳ�����ͬ)��Nԭ�Ӻ˵��������ԭ�Ӱ뾶��С��ԭ�Ӻ˶������ӵ���������ǿ bc 4NH3(g) + 6NO(g) = 5N2(g) + 6H2O(l) ��H = (3c-3a-2b) kJ��mol-1 2HSO3- + 2e- + 2H+ = S2O42- + 2H2O 2NO + 2S2O42- +2H2O = N2 + 4HSO3-
��������
(1)�����ڴ�����������������Ӧ����һ��������ˮ��Ϊ��Ҫ�Ĺ�ҵ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2 4NO+6H2O��
4NO+6H2O��
(2)����C��Nԭ����ͬһ����(����Ӳ�����ͬ)��Nԭ�Ӻ˵��������ԭ�Ӱ뾶��С��ԭ�Ӻ˶������ӵ���������ǿ������Nԭ����������������ǿ��
(3)a��������������ԭ��֮��Ϊ��������a����
b����������ͼ����֪����ͼ�ڱ�ʾN2��H2�������ڴ������棬��ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ�����ѣ��ϼ���������������ͼ����ͼ����Ҫ������������b��ȷ��
c���ڻ�ѧ�仯�У������Ӻ�������ڴ����������¶��ѳ���ԭ�Ӻ͵�ԭ�ӣ�������ѧ���Ķ��ѣ�Ȼ��ԭ����������ϳ��µķ��ӣ��γ��µĻ�ѧ�������Ըù��̱�ʾ�˻�ѧ�仯�а����ɻ�ѧ���Ķ��Ѻ��»�ѧ�������ɣ���c��ȷ��
��ѡbc��
(4)����NH3����ķ�ӦΪ��4NH3(g) + 6NO(g) = 5N2(g) + 6H2O(l)��
��֪��N2(g) �� O2(g) = 2NO(g) ��H = a kJ��mol-1 i��
N2(g) �� 3H2(g) = 2NH3(g) ��H = b kJ��mol-1 ii��
2H2(g) �� O2(g) = 2H2O(l) ��H = c kJ��mol-1 iii��
���ݸ�˹����iii��3- i��3-ii��2�ɵ�4NH3(g) + 6NO(g) = 5N2(g) + 6H2O(l) ��H=(3c-3a-2b)kJ��mol-1��
(5)������������ԭ��Ӧ����ͼ��֪������������ӵõ��ӱ���ԭ����S2O42-���������Һ�������ԣ����Ե缫��ӦʽΪ��2HSO3-+2e-+2H+=S2O42-+2H2O��
�ھ�ͼ��֪S2O42-��һ����������������ԭ��Ӧ�����ɵ�������������������ݵ�ʧ�����غ㡢ԭ���غ�͵���غ㣬��Ӧ�����ӷ���ʽΪ��2NO+2S2O42-+2H2O=N2+4HSO3-��


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶�ʱ����2.0 L�����ܱ������г���2 mol SO2��1 mol O2��������Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
2SO3(g)������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
t / s | 0 | 2 | 4 | 6 | 8 |
n(SO3) / mol | 0 | 0��8 | 1��4 | 1.8 | 1.8 |
����˵����ȷ����( )
A. ��Ӧ��ǰ2 s ��ƽ������v(O2) �� 0��4 mol��L��1��s��1
B. ���������������䣬���ѹ����1.0 L��ƽ�ⳣ��������
C. ��ͬ�¶��£���ʼʱ�������г���4 mol SO3���ﵽƽ��ʱ��SO3��ת����С��10%
D. �����¶Ȳ��䣬����������ٳ���2 mol SO2��1 mol O2����Ӧ�ﵽ��ƽ��ʱn(SO3)/n(O2)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����н�����ʵ�ķ���ʽ��ȷ����
A.��ҵұ���Ȼ������ȵ��ʣ�2AlCl3(����)![]() 2Al+3Cl2��
2Al+3Cl2��
B.���Ȼ�����Һ�м��������ˮ��������ɫ������Al3+ + 3OH- = Al(OH)3��
C.��������ˮ�������ȣ��������壺2Fe��3H2O(g)![]() Fe2O3��3H2
Fe2O3��3H2
D.��ⱥ���Ȼ�����Һ���������壺2NaCl+2H2O![]() 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��HI�������л���Ӧ�еĻ�ԭ�������Ȼᷢ���ֽⷴӦ����֪![]() ʱ��
ʱ��![]() ����1L�ܱ������г���1molHI��
����1L�ܱ������г���1molHI��![]() ʱ����ϵ��
ʱ����ϵ��![]() �뷴Ӧʱ��t�Ĺ�ϵ��ͼ��ʾ������˵���У���ȷ����
�뷴Ӧʱ��t�Ĺ�ϵ��ͼ��ʾ������˵���У���ȷ����

A.![]() min�ڵ�ƽ����Ӧ���ʿɱ�ʾΪ
min�ڵ�ƽ����Ӧ���ʿɱ�ʾΪ![]()
![]()
B.�����¶ȣ��ٴ�ƽ��ʱ��![]()
![]()
C.�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ![]()
D.��Ӧ����40minʱ����ϵ���յ�����ԼΪ![]() kJ
kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ҩƷ��ǩ���йط�������ȷ���ǣ��� ����
ѡ�� | A | B | C | D |
��Ʒ��ǩ | ������ˮ1.01��105 Pa��20 �� | ҩƷ��������
| ̼������NaHCO3����С�մ�84 g��mol��1�� | Ũ����H2SO4 �ܶ�1.84 g��mL��1Ũ��98.0% |
���� | ���Լ�Ӧװ��������ϸ��ƿ�� | ��ҩƷ������Ƥ��ֱ�ӽӴ� | �����������ֽ� | ��ҩƷ��ǩ�ϻ�����
|
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾ�������������ǣ��� ����

A. Ba2����Mg2����NO3-��CO32-B. H����Ba2����Al3����Cl��
C. K����Ba2����Cl����HCO3-D. NH4+��Ba2����Fe2����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ���������[Co(NH3)6]Cl3 �dzȻ�ɫ������ˮ�������Ǻϳ�����һЩ����������ԭ�ϡ���ͼ��ij����С���Ժ��ܷ��ϣ�������Fe��Al �����ʣ���ȡ[Co(NH3)6]Cl3 �Ĺ������̣�
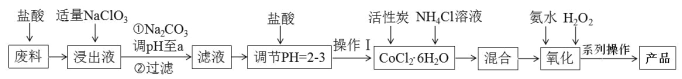
�ش��������⣺
��1��д���ӡ�����NaClO3��������Ӧ�����ӷ���ʽ______________��
��2������Na2CO3 ��pH��a�����������ֳ������ֱ�Ϊ_______________________���ѧʽ����
��3��������IJ������_____________________________����ȴ�ᾧ����ѹ���ˡ�
��4��������NH4Cl������Ӧ���⣬���ɷ�ֹ�Ӱ�ˮʱc(OH��) ������ԭ����_________________��
��5�������������裬��ͬѧ��ΪӦ�ȼ��백ˮ�ټ���H2O2����ͬѧ��Ϊ�Լ�����˳��Բ�����Ӱ�졣����Ϊ___________����ס����ҡ���ͬѧ�۵���ȷ��������_________________________________��д���ò���Ļ�ѧ����ʽ��________________________________
��6��ͨ���������ɲⶨ��Ʒ���ܵĺ������� [Co(NH3)6]Cl3 ת����Co3���������KI ��Һ������Na2S2O3��Һ�ζ�(������Һ��ָʾ��)����Ӧԭ����2Co3����2I����2Co2����I2��I2��2S2O32����2I����S4O62����ʵ������У����в����ᵼ�������ܺ�����ֵƫ�ߵ���_______��
a���þ����ڿ����е� KI ����������Һ
b��ʢװNa2S2O3��Һ�ļ�ʽ�ζ���δ��ϴ
c���ζ��������ֵζ�����������
d����Һ��ɫ��ȥ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������п������п����п�ε���Ҫԭ�ϣ�Ҳ������ʪ�������п����ͼΪ��п����ZnSO4��7H2O����Ĺ������̡�

��֪��
��п�ҵ���Ҫ�ɷ�ΪZnO��������CuO��PbO��MnO��FeO��
��������2������Ҫ�ɷ�ΪFe(OH)3��MnO(OH)2��
��ش��������⣺
��1��MnO(OH)2��MnԪ�صĻ��ϼ�Ϊ___��
��2��Ϊ��߽���Ч�ʣ�п�����������ǰ�ɲ�ȡ�Ĵ�ʩ��___���������ʱ��������Ũ�ȹ��ߣ����ܷ�������Ӧ�Ļ�ѧ����ʽΪ___��
��3��������1������Ҫ�ɷ�Ϊ___��
��4����������ʱ���������Һ��pH=5.1��Fe2�������������ӷ���ʽΪ___��
��5������aΪ___�����ˡ�ϴ�ӡ����
��6��ZnSO4��7H2O��Ʒ�Ĵ��ȿ�����λ�ζ����ⶨ��
ȷ��ȡһ������ZnSO4��7H2O�������250mL����ƿ�У���ˮԼ20mL���ټ���2-3��5%�Ķ��ӳ���ָʾ����Լ5mL���Ǽ��İ�������Һ��ҡ�ȡ����ѱ궨��0.0160mol��L-1EDTA��Һ�ζ����ζ�����Һ�ɺ���ɫ�������ɫ����Ϊ�յ�(ZnSO4��7H2O��EDTA�����ʵ���֮��1��1��Ӧ)��ʵ���������±���

ZnSO4��7H2O��Ʒ�Ĵ���Ϊ___ (����2λ��Ч����)��
��7����ҵ�ϲ��ö��Ե缫���������ZnSO4��Һ��ʵ��ʪ����п���������е����ӷ���ʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���A����Ȼ�ĵ��塣�л���F��һ�ֻ��������ϣ�һ�ֺϳ�·����ͼ��ʾ��
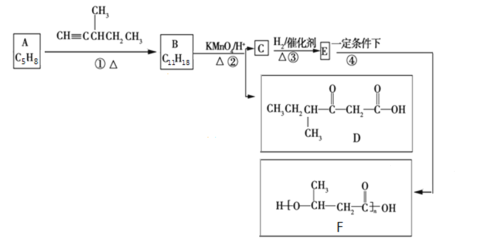
��֪��
��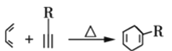
��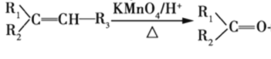 +R3-COOH����R1����R2����R3����R��������
+R3-COOH����R1����R2����R3����R��������
������������⣺
(1)A��ϵͳ����������Ϊ__________________________��
(2)A������Cl2���ӷ���1��1�ӳ�ʱ�������������____________��(�����������칹)��
(3)B������H2��Ӧ��IJ���Ľṹ��ʽΪ![]() ����B�Ľṹ��ʽΪ___________________��1��B��������________������̼ԭ�ӡ�
����B�Ľṹ��ʽΪ___________________��1��B��������________������̼ԭ�ӡ�
(4)C�����еĹ���������Ϊ_____________________��
(5)д����Ӧ�ܵĻ�ѧ����ʽ��______________________________________________________��
(6)G��C��ͬ���칹�壬G�ܷ���ˮ�ⷴӦ��������Ӧ��1��G�����к���2��̼��˫������G�Ŀ��ܽṹ����___________��(�����������칹)��
(7)������л���D�ͼ״�Ϊ��ʼԭ���Ʊ�CH3CH2CH(CH3)CH =CHCOOCH3�ĺϳ�·��______________(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com