
I. ����H2��Cl2�����100 mL�������������һ����������Ϊ45mL������ʹ�������巢����Ӧ��ָ���������������Ϊ mL��Ϊ��˵����Ӧ��������H2��Cl2��ʣ�࣬ʹ����ͨ��10 mLˮ����ʹʣ����������ָ��������
��1������ʣ��___mL��֤����___ʣ�࣬������_____��
��2������Һ��___���ʣ�֤����___ʣ�࣬������________________________��
II.һλͬѧ�����һ����Ũ�����KMnO4������ȡ�����������Ƚ�������ⵥ�ʵ�������ǿ������װ�ã���ͼ��ʾ����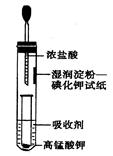
��1��������Һ������Cl2����________��
| A������ʳ��ˮ | B��Na2SO4��Һ |
| C��NaOH��Һ | D��Ũ���� |
��100(2��) ��1��10��H2��H2������ˮ
��2��Ư�ף�Cl2��Cl2����ˮ��������Ư���Ե�HClO (ÿ��1��)
��(ÿ��2��)(1)C
(2)ʪ����ۡ��⻯����ֽ����
(3)MnO2+4H++2Cl�� Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O
��������������������������ķ�Ӧ�Ƿ�Ӧǰ����������ޱ仯�ķ�Ӧ�����Է�Ӧ�����������Ϊ100ml��
��1������10ml����������������Ϊ����������ˮ��
��2������Һ��Ư���ԣ���֤��������ʣ�࣬��Ϊ��������ˮ�д��������ɣ����������Ư���ԡ�
��1����������ˮ����Һ�����ԣ����Ӧ�ü�����Һ���գ�ѡC��
��2��ʪ����ۡ��⻯����ֽ����˵���������û����⣬�Ӷ���˵��Cl2��������ǿ��I2��
��3��ʵ��������Ũ������������̹����������������ӷ���ʽΪ��MnO2+4H++2Cl�� Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O
���㣺������������ȡ�������Ե��жϡ���ˮ�����ʡ������������ˮ�ķ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��X��Y��Z��W���ֶ���������Ԫ�أ�A�ǵؿ��к������Ľ���Ԫ�أ�����������Ԫ����B��ԭ�Ӱ뾶���X��Y��Z��WԪ�������ڱ��е����λ������ͼ��ʾ������ZԪ��ԭ�������������ǵ��Ӳ�����2������ش�����
���⣺ 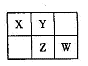
��1��W����������ﻯѧʽ�� ��Z��ԭ�ӽṹʾ��ͼΪ ��
��2��A��B��������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ ��
��3��AW3�����ھ�ˮ����ԭ���� �����������ӷ���ʽ��ʾ��
��4����ҵ�ϳ�X�ļ���̬�⻯���Ƿ��ȷ�Ӧ�����д�ʩ�м������
��Ӧ���ʣ��������ԭ��ת���ʵ��� ��
a�������¶�
b���������
c����X�ļ���̬�⻯�Pʱ����
d������Ӧ��ϵ��ѹǿ
��5����״���£�2.24L X�ļ���̬�⻯�ﱻ200 mL l mol L��1X������������Ӧ��ˮ������Һ���պ�������Һ������Ũ�ȴӴ�С��˳���ǣ������ӷ��ű�ʾ�� ��
��6��WY2��ɱ��������ͬʱ���ɽ��綾�軯�����������������ȥ��д����WY2���е�9.9�棩������ȥCN�������ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013��ף��Ϻ����ַ���һ��������ʳ��������������к������IJҾ硣����������������Ҫ���������ƣ�NaNO2��������һ�ְ�ɫ�������壬��Ȼ��״����ʳ�Σ���������ζ�����ж����������ƺ��Ȼ��ƵIJ����������±���
| | �������ƣ�NaNO2�� | �Ȼ��ƣ�NaCl�� |
| ˮ���� | ���ܣ���Һ�������� | ���ܣ���Һ������ |
| �۵� | 271�� | 801�� |
| �е� | 320���ֽ� | 1413�� |
| ��ϡ�������� | �к���ɫ��NO2����ų� | ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ˮ��Һ���в�ͬ����Ϊ����Ҫ��ش��������⣺
��1��Na2SO3��Һ�Լ��ԣ���ԭ����______________________________(�����ӷ���ʽ��ʾ)������Һ�и�����Ũ���ɴ�С��˳��Ϊ______________________��
��2����������10mL��ˮ��Һ�м�ˮϡ�ͺ��������������__________(���ţ���ͬ)����С����___________���������____________��
a����Һ������Ũ�� b����ˮ�ĵ���̶�
c��ˮ�����ӻ����� d��c(H+)/ c(NH3��H2O)
��3���������ƣ�NaClO2����һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ�������ѱ��ʺ�δ���ʵ�NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2+�����ʵ��� �����ͬ������ͬ��������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֡�
��1�����ü������ԭ���������֪��
CH4 (g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H ����574 kJ/mol
CH4(g)��4NO(g) �� 2N2(g)��CO2(g)��2H2O(g) ��H ����1160 kJ/mol
��CH4 ��NO2 ��ԭΪN2 ���Ȼ�ѧ����ʽΪ ��
��2������NH3����ԭ�������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ��2NH3(g)��NO(g)��NO2(g)  2N2(g)��3H2O(g)����H < 0
2N2(g)��3H2O(g)����H < 0
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
��3������ClO2�������������ת���������£�
NO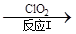 NO2
NO2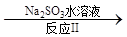 N2
N2
��֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O �� NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
��4������CO����ԭ��������Ҳ���Դﵽ������Ⱦ��Ŀ�ġ�
��֪����һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ�Ƿ�Ӧ2NO(g) + 2CO(g) 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������������(S)��ʱ��(t)�ı仯���ߡ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)�����������S1 S2 (�����������������ȷ����)��
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������������(S)��ʱ��(t)�ı仯���ߡ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)�����������S1 S2 (�����������������ȷ����)��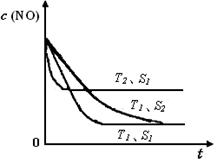
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������(K2FeO4)��һ�����͡���Ч�������ɫˮ����������Cl2��O2��ClO2��KMnO4�����Ը�ǿ��������Ⱦ����ҵ�������Ƶø������ƣ�Ȼ���ڵ����£������������Һ�м���KOH�����ͣ�ʹ�������������
(1)�ɷ��Ʊ�������ص���Ҫ��ӦΪ��2FeSO4�� 6Na2O2=2Na2FeO4�� 2Na2O �� 2Na2SO4�� O2��
�ٸ÷�Ӧ�е���������________����ԭ����________��ÿ����1 mol Na2FeO4ת��________mol���ӡ�
�ڼ�Ҫ˵��K2FeO4��Ϊˮ������ʱ���������__________________________________
(2)ʪ���Ʊ��������(K2FeO4)�ķ�Ӧ��ϵ�����������ӣ�Fe(OH)3��ClO����OH����FeO42����Cl����H2O��
��д������ƽʪ���Ƹ�����ط�Ӧ�����ӷ���ʽ��____________________________
________________________________________________________________________��
��ÿ����1 mol FeO42��ת��________mol���ӣ�����Ӧ������ת����0.3 mol���ӣ���ԭ��������ʵ���Ϊ________mol��
�۵����£��ڸ���������Һ�м���KOH�����Ϳ������������(K2FeO4)��˵��ʲô����_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.ijʵ��AС����������������ƣ�NaClO2������Ҫ������ͼ��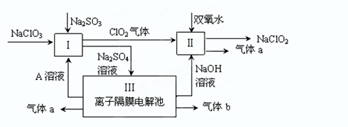
��֪NaClO2��һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ҵ�����ڼ��Ի������ȶ����ڡ�
��1��˫��ˮ�ĵ���ʽΪ ��װ�â��з�����Ӧ�Ļ�ԭ�����������������ѧʽ����
��2��A�Ļ�ѧʽ������ ����װ�â������A��������������������װ�â�����������aΪ 11.2 L(��״��)����������ͨ�����صĵ���Ϊ������ ����֪�����ڳ���F="9.65��l" 04C�� mol-1)��
��3��װ�â��з�Ӧ�����ӷ���ʽ������������ ������ ��
��ijʵ��BС��ⶨ�������Ͻ���Ʒ�Ĵ��ȣ���������п��ͭ����ɾ��ȣ�������Ʒ������������: Sn+ 2HCl=SnCl2+H2�������ˣ�ϴ�ӡ�����Һ��ϴ��Һ�ϲ��ټӹ�����FeCl3��Һ��������һ��Ũ�ȵ�K2Cr2O7������Һ�ζ����ɵ�Fe2+����ʱ��ԭ����ΪCr3+���������Ͻ�����1.23g����������Ӧ����������ȥ0.200mol/L��K2Cr2O7��������Һ15.00mL��
��4�� ��ʽ������Ʒ����������������
��5����������Ʒģ�ҵ�ϵ�⾫��������ͼ��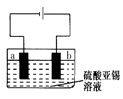
b�������缫��Ӧʽ �����õ�11.90g����ʱ���������Һ��������0.54g�������Ͻ���������_______ g���������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о�̼���仯������ۺ����öԴٽ���̼���Ĺ���������Ҫ�����塣���������֪ʶ�о�̼���仯��������ʡ�
��1�����������ҹ���������̼���о�ȡ���ش��չ���õ绡���ϳɵ�̼�����г����д���̼�����������ʣ�������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ��______C��________K2Cr2O7��________����________CO2����________K2SO4��________Cr2��SO4��3��________H2O��
����ɲ���ƽ������ѧ����ʽ��
����������ѧ����ʽ�ϱ���÷�Ӧ����ת�Ƶķ�������Ŀ��
��2������ʱ����CO��ԭMgSO4���Ʊ��ߴ�MgO��
��750��ʱ����������к������ʵ���SO2��SO3����ʱ��Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
����MgO���Ƴɡ�þ���������Ρ�ȼ�ϵ�أ���װ��ʾ��ͼ��ͼ��a����ʾ���õ�ط�Ӧ�����ӷ���ʽΪ________________________________________��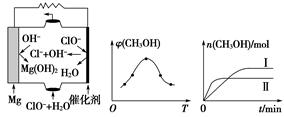
��a���������� ����b��������������c��
��3��������̼�ϳɼ״���̼���ŵ��·���CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪCO2��g����3H2��g��  CH3OH��g����H2O��g������H��
CH3OH��g����H2O��g������H��
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK��________��
��ȡ��ݵ����CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״�����������գ�CH3OH���뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��b����ʾ��������CO2ת��Ϊ�״���Ӧ�Ħ�H________�������������������0��
�������ֲ�ͬ�����·�����Ӧ�����CH3OH�����ʵ�����ʱ��仯��ͼ��c����ʾ�����ߢ��Ӧ��ƽ�ⳣ����С��ϵΪK��________K�����������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ���ҿ���KMnO4�����Ũ���ᷴӦ����������Ӧ�Ļ�ѧ����ʽ���£�
2KMnO4+16HCl(Ũ) =2KCl+2MnCl2+5Cl2��+8H2O��
��1���÷�Ӧ����������_____________����ԭ����__________________��
��2������Ӧ����0.20 mol ���ӷ���ת�ƣ��������������Ϊ ����״��������������HCl�����ʵ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com