
����Ŀ���״�������������ϸ���У���ά�ֹ��������ݵı�Ҫ���ʣ������������������ϵĻ�ѧ��Ӧ�������������й��ɵ�������ά�������������ܺʹ����̼�����Ҫ���ʡ������Ļ������ǹ�ҵ�ϵ���Ҫԭ�ϡ������������գ�
P(s������)+![]() O2(g)=
O2(g)=![]() P4O10(s)+738.5 kJ
P4O10(s)+738.5 kJ
P4(s������)+ 5O2(g)=P4O10(s)+2983.2 kJ
(1)�����ת��Ϊ�����Ȼ�ѧ����ʽ_____________________��
(2)������KOH��Һ������Һ�к�KOCl���ã���������K6P6O12�ļ��κ�KCl�Ȳ����д���÷�Ӧ�Ļ�ѧ����ʽ�����������ת�Ʒ������Ŀ��____
(3)�Ʊ�MgNH4PO4ʱͨ����þ�Σ����Ȼ�þ����Һ�м�Na2HPO4 ����ˮ�������Һ�����Ȼ�泥�����ӦʽΪMgCl2+Na2HPO4+NH3=2NaCl+MgNH4PO4�����ڴ˷�Ӧ�У����������Σ��ڼ�����Һ�У�Mg2+���ᱻ��ˮ�����������ӷ���ʽΪ____________________���������֮�ɷ�ֹ����Mg(OH)2���������õ���ƽ��ԭ������ԭ��_________
(4)H3PO3���ˮ��Ӧ����ˮ���ػ�ɫ��ȥ���������ᣬ��������Ӧ�����Һ�м��������AgNO3��Һ�����ɻ�ɫ��������H3PO3���ˮ��Ӧ�Ļ�ѧ����ʽΪ___________����ɫ������_________����������_____________________��д������һ����;���ɣ���
���𰸡�P4(s������)=4P(s������) +29.2kJ ![]() Mg2++2NH3.H2O=Mg(OH)2��+2NH4+ �������֮��������Һ��笠����ӵ�Ũ������ʹƽ��NH3.H2ONH4++OH-�����ƶ����������Һ��OH-Ũ��(����NH4+Ũ�ȣ�����NH3.H2O����) H3PO3+I2+H2O=H3PO4+2HI AgI �������������Ƭ���ĸй�㡢�˹�����
Mg2++2NH3.H2O=Mg(OH)2��+2NH4+ �������֮��������Һ��笠����ӵ�Ũ������ʹƽ��NH3.H2ONH4++OH-�����ƶ����������Һ��OH-Ũ��(����NH4+Ũ�ȣ�����NH3.H2O����) H3PO3+I2+H2O=H3PO4+2HI AgI �������������Ƭ���ĸй�㡢�˹�����
��������
(1)���ø�˹���ɼ���õ��Ȼ�ѧ����ʽ��
(2)��������д����ѧ����ʽ�����û��ϼ۵ı仯������ӵ�ת����Ŀ��
(3)þ���ӺͰ�ˮ��Ӧ����������þ��笠����ӣ�д����ѧ����ʽ��������Σ�����笠����ӵ�Ũ�ȣ����ð�ˮ�ĵ���ƽ���ƶ�������
(4)H3PO3���ˮ��Ӧ����ˮ���ػ�ɫ��ȥ���������ᣬ�ⱻ��ԭΪ�⻯�⣬д����ѧ����ʽ���⻯��������������ɵĵ⻯���ǻ�ɫ���������õ⻯������;�ش�
(1)P(s������)+![]() O2(g)=
O2(g)=![]() P4O10(s)H1=+738.5 kJ/mol ��
P4O10(s)H1=+738.5 kJ/mol ��
P4(s������)+ 5O2(g)=P4O10(s)H2=+2983.2 kJ/mol ��
��-����4�õ�P4(s������)=4P(s������) H=-29��2kJ/mol ��
(2)������KOH��Һ������Һ�к�KOCl���ã���������K6P6O12��KCl��ˮ����ѧ����ʽΪ6P+9KOCl+6KOH= K6P6O12+9KCl+3H2O���õ����ű�ʾ�����ӵ�ת�ƺͷ������ǻ�ԭ�������ϼ۴�0���ߵ�+3�ۣ���6����ԭ�����˻�ԭ����KOCl������������ԭ�Ӵ�+1�۽��͵�-1�ۣ���ת�Ƶĵ�����Ϊ18�� ![]()
(3)þ���ӻ�Ͱ�ˮ��Ӧ����������þ��笠����ӣ����ӷ���ʽΪMg2++2NH3.H2O=Mg(OH)2��+2NH4+���������֮��������Һ��笠����ӵ�Ũ������ʹƽ��NH3.H2ONH4++OH-�����ƶ����������Һ��OH-Ũ�ȣ�����NH4+Ũ�ȣ�����NH3.H2O���룩��
(4)H3PO3���ˮ��Ӧ����ˮ���ػ�ɫ��ȥ����������͵⻯�⣬��ѧ����ʽΪH3PO3+I2+H2O=H3PO4+2HI���⻯������������Ӧ���ɵ⻯���͵⻯�⣬�⻯���ǻ�ɫ���������������������Ƭ���ĸй�㡢�˹����ꡣ


 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����״�л���A��һ��ʳ�����㾫����һ������������仯��

��֪��(i) ![]()
(ii)A��G��Ϊͬ���칹�壬A����ʹBr2��CCl4��Һ��ɫ��B��F�����������ŵ�������ͬ��
���������գ�
(1)F�ķ���ʽΪ___________��CD���Լ���������________________��
(2)A�Ľṹ��ʽΪ_______________�� BH�ķ�Ӧ������_____________��
(3)I������̼ԭ�Ӿ���һ��ֱ���ϣ�Hת��ΪI�Ļ�ѧ����ʽΪ_______________��
(4)X��A��һ��ͬ���칹�壬1 mol X ��HIO4������������ȫ��Ӧ����������1 mol��֧�����л����X�Ľṹ��ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ԭ������Ȼ��һ����Ҫ�Ļ���ԭ��,������ʵ������������ԭ�����͵���
A. NO2������ѹ������ɫ�ȱ�����dz
B. ��2HI(g) ![]() H2(g)��I2(g)ƽ����ϵ��ѹ����ɫѸ�ٱ���
H2(g)��I2(g)ƽ����ϵ��ѹ����ɫѸ�ٱ���
C. �ϳɰ���ҵ���ø��¡���ѹ������߰��IJ���
D. ��������������Һʱ��������������м�Է�ֹ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������һ�����Ƴ���������ҩ����ɰ�����(![]() )�ͼ״���Ӧ�Ƶá����������գ�
)�ͼ״���Ӧ�Ƶá����������գ�
(1)д������������Ľṹ��ʽ��_______________________________��
(2)��ҵ����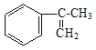 �����IJ���ͬ���͵ķ�Ӧ�Ƶð����ᡣ�밴ʵ�ʽ��еķ�Ӧ˳��д��ָ����Ӧ����������Ҫ���Լ��ͷ�Ӧ������
�����IJ���ͬ���͵ķ�Ӧ�Ƶð����ᡣ�밴ʵ�ʽ��еķ�Ӧ˳��д��ָ����Ӧ����������Ҫ���Լ��ͷ�Ӧ������
��һ��________________________________�� �ڶ���________________________________��
(3)д�����IJ���Ӧ�Ļ�ѧ����ʽ_______________________________________________��
(4)A�DZ� ������̼ԭ�ӵ�һ��ͬϵ���A������̼ԭ�ӿ��Դ���ͬһƽ���ϡ�д��A�Ľṹ��ʽ________________________________��
������̼ԭ�ӵ�һ��ͬϵ���A������̼ԭ�ӿ��Դ���ͬһƽ���ϡ�д��A�Ľṹ��ʽ________________________________��
(5) ![]() �ǰ����������ij��ͬ���칹���һ�ȴ��������������������Һ���ȣ����ɵ��л�����Ľṹ��ʽΪ_____________________________________��
�ǰ����������ij��ͬ���칹���һ�ȴ��������������������Һ���ȣ����ɵ��л�����Ľṹ��ʽΪ_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ�������Դ����ˮú����ȡ���ѵ�ԭ�����£�
������2H2(g)+CO(g)![]() CH3OH(g) ��H1
CH3OH(g) ��H1
������2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H2
CH3OCH3(g)+H2O(g) ��H2
��1����4H2(g)+2CO(g)![]() CH3OCH3(g)+H2O(g)����H=___���ú���H1����H2��ʽ�ӱ�ʾ��
CH3OCH3(g)+H2O(g)����H=___���ú���H1����H2��ʽ�ӱ�ʾ��
��2���ں����ܱ������з�����Ӧ��������
������ͼ����ȷ��ӳƽ����ϵ�м״�����������¶ȱ仯�����������__������a������b������
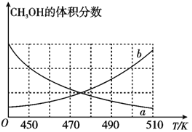
��������˵���ܱ�����Ӧ�������Ѵ�ƽ��״̬����__��
a.�����������ѹǿ���ٱ仯 b.���������ܶȲ��ٱ仯
c.��������ƽ����Է����������ٱ仯 d.v����H2��=2v����CH3OH��
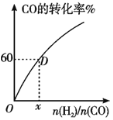
����300���£�CO��ת��������ʼͶ�ϱ�![]() �ı仯��ϵ��ͼ��ʾ�����D��������ת����Ϊ40%��x=__��
�ı仯��ϵ��ͼ��ʾ�����D��������ת����Ϊ40%��x=__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
��֪��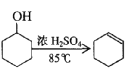 +H2O
+H2O
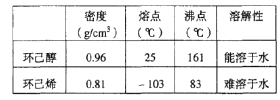
(1)��ͼ1��ʵ������ȡ����������װ�á�������˵������ȷ������____��
A���ұߵ��ܲ����뱥��̼������Һ
B���Թ�D����״�����²㣬����̼������Һ��ҪΪ���кͻӷ���������
C���Թ�C�м����Լ���˳���ǣ�2mLŨ������3mL�Ҵ���2mL������
D����Ӧ�������Թ�CҺ����ܻ���ɫ
(2)����ȡ���������Ĺ����У���Ũ������Ϊ������ȱ�㣬�����ظ�ʹ�ã����Ҹ���Ӧ�϶ࡣĿǰ�Ը÷�Ӧ�Ĵ����������µ�̽����������������������Һ��������˷�Ӧ�Ĵ����������ظ�ʹ�á�ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ���
ͬһ��Ӧʱ�� | ͬһ��Ӧ�¶� | ||||
��Ӧ�¶�/�� | ת����(%) | ѡ����(%)* | ��Ӧʱ��/h | ת����(%) | ѡ����(%)* |
40 | 77.8 | 100 | 2 | 80.2 | 100 |
60 | 92.3 | 100 | 3 | 87.8 | 100 |
80 | 92.6 | 100 | 4 | 92.3 | 100 |
120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
ѡ����100%��ʾ��Ӧ���ɵIJ���������������ˮ | |||||
���ݱ������ݣ�����________������ĸ��Ϊ�÷�Ӧ�����������
A.120��,4h B.80��,6h C. 60��,4h D.80��,4h
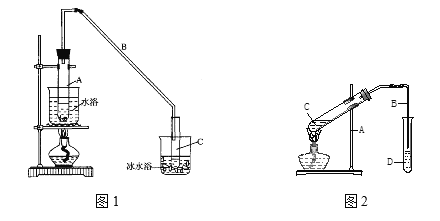
(3)�Ʊ�����ϩ��Ʒ��װ����ͼ2��
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_____________��
(4)�Ʊ�����ϩ��Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һʱ��������Һ�����������������ԭ�����Һ©�����������⣬����________����Һ����_________ (������)ϴ�ӣ�
a��NaHSO4��Һ b��Na2CO3��Һ c��ϡH2SO4 d.��ˮ
�ڴ��Ʊ������У����뱥��ʳ��ˮ��������__________________��
�ڶԷ�������Ļ���ϩ�ٽ�������õ�����ϩ��Ʒ��Ϊ�����ֻ���ϩ��Ʒ�ʹ�Ʒ��ij��С����������¼��ַ�������������_________��
a�������Ը��������Һ b���ý���K c����Na2CO3��Һ d.��NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ����
A����֪ P(s,����) =P(s,����) ��H<0,����ױȺ����ȶ�
B����֪ 2H2(g)+O2(g)=2H2O(g) ��H=-483.6 kJ��mol-1,��������ȼ����Ϊ 241.8 kJ��mol-1
C����֪ 2C(s)+2O2(g)=2CO2(g) ��H=a 2C(s)+O2(g)=2CO(g) ��H=b,�� a>b
D����֪ NaOH(aq)+HCl(aq)= NaCl(aq)+H2O(l) ��H=-57.3 kJ��mol-1,�� 40.0 g NaOH��ϡ��Һ��ϡ������ȫ�к�,�ų�С��57.3 kJ ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�߷����л���P�ĺϳ�·�����£�
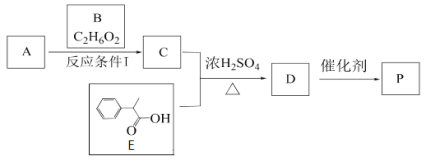
��֪1 mol B�������Ľ����Ʒ�Ӧ���������22.4L����
(1)A��2-����ϩ�ᣬA�Ľṹ��ʽ��______________��E�Ļ�ѧʽΪ___________________
(2)A��B����C�ķ�Ӧ������________��D����P�ķ�Ӧ������______________
(3)A�������B��Ӧ����C����A��������õ�C��, C���Ľṹ��ʽΪ__________________
(4)P��NaOH��Һ��������ȫˮ��Ļ�ѧ����ʽ�ǣ�_________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ե��з�̪��Һ��������Һ����������ɫ�������
A. ������Һ���� B. CH3COONa��Һ����
C. ��ˮ�м�������NH4Cl���� D. С�մ���Һ�м�������NaCl����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com