
����Ŀ������ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ�����һ�µ���![]()
ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
A | �������� | ��ȥ |
B | ��ij��Һ�м��� | ֤����Һ�к� |
C | ��ij��Һ�м���ϡ���ᣬ�ų���ɫ��ζ���壬������ͨ�����ʯ��ˮ��ʯ��ˮ����� | ֤������Һ�д��� |
D | �� | ֤�� |
A.AB.BC.CD.
���𰸡�A
��������
A����������![]() ��
��![]() ��Һ�м�������
��Һ�м�������![]() ��ĩ������һ��ʱ�����ˣ��ٽ������ӵ�ˮ�⣬ʹ��ת��Ϊ�������ﵽ���ӵ�Ŀ�ģ���A��ȷ��
��ĩ������һ��ʱ�����ˣ��ٽ������ӵ�ˮ�⣬ʹ��ת��Ϊ�������ﵽ���ӵ�Ŀ�ģ���A��ȷ��
B�����ᱵ��AgCl��Ϊ��������İ�ɫ����������ij��Һ�м���![]() ��Һ���ɰ�ɫ������������ϡ�����������ʧ����Һ�п��ܺ�
��Һ���ɰ�ɫ������������ϡ�����������ʧ����Һ�п��ܺ�![]() ����B����
����B����
C����ij��Һ�м���ϡ���ᣬ�ų���ɫ��ζ���壬������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�����Ϊ������̼����Ϊ̼���λ�̼��������Һ����C����
D����![]() ��Һ�еμ���������
��Һ�еμ���������![]() ��Һ��
��Һ��![]() ��Һ��ɫ����������ԭ��Ӧ��FeԪ�صĻ��ϼ����ߣ���֤��
��Һ��ɫ����������ԭ��Ӧ��FeԪ�صĻ��ϼ����ߣ���֤��![]() ���л�ԭ�ԣ���D����
���л�ԭ�ԣ���D����
��ѡA��


 ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵������ȷ����
A.C3H8��̼ԭ�Ӷ����õ���sp3�ӻ�
B.O2��CO2��N2���ǷǼ��Է���
C.���ԣ�H2CO3��H3PO4��H2SO4��HClO
D.CO��һ�ֵȵ�����ΪNO+�����ĵ���ʽΪ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������ԭ�ӻ����ӵĵ����Ų��ı�ʾ�����У���ȷ����___��Υ�����������ԭ������___��Υ�����ع������__��
��Ca2����1s22s22p63s23p6
��F����1s22s23p6
��P��![]()
��Cr��1s22s22p63s23p63d44s2
��Fe��1s22s22p63s23p63d64s2
��Mg2����1s22s22p6
��C��![]()
����ʯ�������鱦�繫��Ϊ�Ĵ�����ʯ֮һ����Ҫ�ɷ�ΪBe3Al2[Si6O18]����������Cr2O3��0.15��0.6%�������γ���ĸ�̡��Իش��������⣺
��2����̬Alԭ���У�������������ܼ���___����̬Crԭ�ӵļ۵����Ų�ʽ��___��
��3��������������������գ�
��һ������ | �е� | ���Ӱ뾶 |
Be___B | H2S___H2O | Al3+___O2- |
��4��߲����Fe2���ϼ����γ�Ѫ���أ�Fe2���ĵ����Ų�ʽΪ___���������ڱ��е�λ��Ϊ__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����ͬpH������һԪ����HA��HB��Һ�ֱ��ˮϡ�ͣ���ҺpH���ˮ����仯��������ͼ��ʾ������˵����ȷ����

A. HB������ǿ��HA
B. a����Һ�ĵ����Դ���b����Һ
C. ͬŨ�ȵ�NaA��NaB��Һ�У�c(A-)=c(B-)
D. ��ˮϡ�͵�pH��ͬʱ����HA��HB�õ���ˮV(A)С��V(B)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������������ء�����˵���������![]()
![]()
A.��ĭ�����������һ������Ҳ�����ڵ������
B.��ɫ��ѧҪ���Դͷ�����������������Ի�������Ⱦ
C.��¯ˮ���к��е�![]() ��������
��������![]() ��Һ�������������ȥ
��Һ�������������ȥ
D.����ˮ����þ����ֹ�ڵ���ʴ��ԭ������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯������Tũҵ����������Ҫ������
(1)![]() �ڿ��������տ��Ƶ���ϵ��������ϡ���֪
�ڿ��������տ��Ƶ���ϵ��������ϡ���֪![]() ��101kPaʱ��
��101kPaʱ��
![]()
![]()
![]()
![]()
д��![]() �ڿ�������������
�ڿ�������������![]() ���Ȼ�ѧ����ʽ______
���Ȼ�ѧ����ʽ______
(2)�����г���CO��ԭ����������ұ������Tҵ�Ͽ����ü�����ˮ������Ӧ�Ʊ�COԭ������![]() ����ͼ��
����ͼ��![]() ��
��![]() ��ʼ�����Ϊ1��3ʱ������ϵ�м��������������¶ȡ�ѹǿӰ��Ĺ�ϵͼ��
��ʼ�����Ϊ1��3ʱ������ϵ�м��������������¶ȡ�ѹǿӰ��Ĺ�ϵͼ��
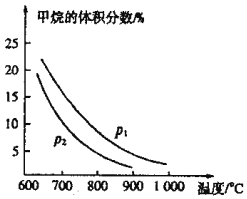
�ټ�����ˮ������Ӧ�Ʊ�CO�ķ�ӦΪ______![]() ��������������������
��������������������![]() ��Ӧ��ѹǿ
��Ӧ��ѹǿ![]() ______
______![]() ����
����![]() ������
������![]() ��
��![]()
���ں��º�ѹ�������£�������Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ�����淴Ӧ�����ƶ�����______
A.�淴Ӧ������������С
B.���������ܶȼ�С
C.��ѧƽ�ⳣ��Kֵ��С
D.ˮ������ת���ʼ�С
���ں��¡����ݵ������£�������ƽ����ϵ�г��������Ϊ��3�ļ�����ˮ����������壬�ٴδﵽƽ��ʱCO���������______![]() ��������������С������������
��������������������������![]() ��
��
(3)��֪![]() ʱ��
ʱ��![]() �����¶��·�Ӧ
�����¶��·�Ӧ![]() ��ƽ�ⳣ��
��ƽ�ⳣ��![]() ______
______
(4)��ҵ��ͨ�����ŨNaOH��Һ���Ʊ�![]() ��Ȼ��ת��Ϊ
��Ȼ��ת��Ϊ![]() �����ԭ����ͼ��ʾ����A��Һ�����ʵijɷ�Ϊ______
�����ԭ����ͼ��ʾ����A��Һ�����ʵijɷ�Ϊ______![]() �ѧʽ
�ѧʽ![]() �������ĵ缫��ӦʽΪ______
�������ĵ缫��ӦʽΪ______
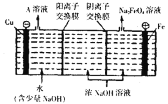 .
.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����Ħ������ԽС����ɢ�ٶ�Խ�졣ͼ��ʾΪ������ɢ�ٶȵ�ʵ�顣����������ɢʱ�γ�ͼʾ�İ�ɫ�̻����Լס������ʵ��жϣ���ȷ����

A.����Ũ��ˮ������Ũ����
B.����Ũ��ˮ������Ũ����
C.��������������Һ������Ũ����
D.����Ũ���ᣬ����Ũ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˼����������Ͷ�����̼�Ļ���������Ƿ������һ����̼�������µ�װ�ý���ʵ�顣��ش�
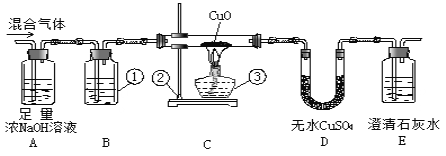
(1)д�����з��ŵ��������ƣ���___________����_____________����_____________��
(2)װ��B���õ��Լ��� _______________��Ŀ����Ϊ��_______________________��
(3)���۲쵽Eװ���г���____________����ʱ��˵�����������һ������һ����̼��
(4)�����������к���һ����̼��Ϊ�˱���������Ӧ��Eװ���ұߵ������ܿڲ�ȡ�Ĵ�ʩ��_____��
(5)Aװ�õ�������___________����Ӧ�Ļ�ѧ����ʽ��___________________��
(6)�����������е�CO��CuO��ȫ��Ӧ����ͨ�������Ϊmg�� D����ng��Eƿ����pg������������CO�������ٷ���Ϊ��_________%�����ȥ��Dװ�ã���������CO�������ٷ���ȷ��Ϊʲô��___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���絼���Ǻ����������Һ����������С��������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㡣��ͼ��ijͬѧ��0.1molL-1KOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1molL-1��HCl��CH3COOH��Һ�ζ�����ʾ��ͼ(�����Һ����仯���Բ���)�������й��жϲ���ȷ����

A������������0.1 molL-1 KOH��Һ�ζ�CH3COOH��Һ�ĵζ�����
B����A�����Һ���У�c(CH3COO-)+c(OH-)-c(H+)�T0.05 molL-1
C����B�����Һ���У�c(K+)��c(OH-)��c(CH3COO-)��c(H+)
D������ͬ�¶��£�C��ˮ�����c(H+)����A��ˮ�����c(H+)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com