
����Ŀ��ijͬѧ�ñ�NaOH��Һ���ⶨδ֪Ũ�ȵ������Ũ�ȣ�
(1)������250mL 0.5mol/L��NaOH����Һ����ij���������NaOHΪ________ g��
��Ҫ����Ҫ���������в����������ձ�����ͷ�ιܡ���Ͳ��___________��
(2)�õζ���ȷ��ȡ20.00mLδ֪Ũ�ȵ���������ƿ�У������̪��ָʾ������NaOH��Һ�ζ����յ㡣����ƿ�Ĵ���Һ�еμ�2��3�η�̪��Һ������ʼ�ζ���
���ۣ����ֿ��Ƶζ��ܻ���������ҡ����ƿ���۾�____________��
���٣��ȿ���������ӽ��յ�ʱ��Ӧһ��һҡ���ζ��յ���жϣ�___________�������յ㣬�����������¼���ݡ�
(3)��ͬѧ����������ʵ�飬ʵ���������±���
ʵ���� | ����������mL�� | ��NaOH��Һ�������mL�� |
�� | ����20.00 | 16.90 |
�� | 17.10 | |
�� | 18.20 |
�ζ������ϴ���ǵ�______��ʵ�顣����������Ŀ���ԭ����__________(��ѡ����)
a.�ζ�����ʢװ��NaOH��Һǰδ��ϴ
b.��ʢװδ֪Ũ�ȵ�����֮ǰ��ƿ��������������ˮ��δ���
c.�ﵽ�ζ��յ�ʱ��������Һ��Һ����͵����
d.�ζ���ʼǰʢװ��NaOH��Һ�ĵζ��ܼ��첿��û�����ݣ��ڵζ��յ����ʱ���ּ��첿��������
e.�ζ������У���ƿҡ����̫���ң�������ЩҺ�ηɽ�����
f.�ζ���ʼǰʢװ��NaOH��Һ�ĵζ��ܼ��첿�������ݣ��ζ��յ����ʱδ��������
(4)��ͬѧ�������������ʵ���Ũ��Ϊ_________��(���������λС��)��
���𰸡�5.0 250mL����ƿ ע����ƿ����Һ����ɫ�仯 ��Һ��ɫ����ɫ���dz��ɫ���Ұ�����ڲ���ɫ �� af 0.425mol/L
��������
(1)����c=![]() �������ʵ����ʵ������ڸ���m=n��M�������ʵ�������
�������ʵ����ʵ������ڸ���m=n��M�������ʵ�������
(2)���ݵζ������������
(3)c(��)=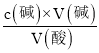 ���жϲ��������������������Ӱ�죻
���жϲ��������������������Ӱ�죻
(4)����c(��)=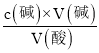 ���㡣
���㡣
(1)һ����������ƿֻ��������Ӧ�������Һ������250mL 0.5mol/L��NaOH����Һ��Ӧѡ��250ml�������ƿ��������Һ��NaOH�����ʵ���n(NaOH)=cV=0.5mol/L��0.25L=0.125mol����������m(NaOH)=0.125mol��40g/mol=5.0g��
һ����������ƿֻ��������Ӧ�������Һ������ƿӦѡ��250ml���һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ò��������裬��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ�Ӳ���ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�����Һ������̶���ˮƽ���У��Ǻ�ƿ����ҡ�ȣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ���Ͳ��(���á�Ҳ�ɲ���)����������250ml����ƿ����ͷ�ιܣ�
(2)�ζ�����ʱ�����ֿ��Ƶζ��ܻ���������ҡ����ƿ���۾�ע����ƿ����Һ����ɫ�仯���μ��ٶ��ȿ���������ӽ��յ�ʱ��Ӧһ��һҡ�����ζ�����Һ��ɫ����ɫ���dz��ɫ���Ұ�����ڲ���ɫ�ﵽ�ζ��յ㣬�����������¼���ݡ�
(3)�Աȱ������ݣ����ϴ���ǵڢ۴�ʵ�飬��Ҫ��Һ�����ƫ����c(��)��V(��)=c(��)��V(��)�жϣ�
a.��ʽ�ζ�����װҺǰδ�ñ�NaOH��Һ��ϴ2��3�Σ���ҺŨ��ƫС�������ƫ��a��ȷ��
b.��װδ֪Ũ������ǰ��ƿ����������ˮ��δ��ɣ���Ӱ�죬b����
c.�ﵽ�ζ��յ�ʱ��������Һ��Һ����͵�������ᵼ�¶���ƫС��Ũ��ƫ�ͣ�c����
d.�ζ���ʼǰ��ʽ�ζ��ܼ��첿��û�����ݣ��ڵζ��յ����ʱ���ּ��첿�������ݣ��ᵼ�����ƫС������Ũ��ƫ�ͣ�d����
e.�ζ������У���ƿҡ����̫���ң�������ЩҺ�ηɽ�������������Ҫ��Һ���ƫС��ʹ�ⶨ��Ũ��ƫ�ͣ�e����
f.�ζ���ʼǰ��ʽ�ζ��ܼ��첿�������ݣ��ڵζ��յ����ʱδ�������ݣ��ᵼ�¶���ƫ�����յ��²ⶨ��Ũ��ƫ�ͣ�f��ȷ��
�ʺ���ѡ����af��
(4)ȡ�١��۴�ʵ���������м��㣬���ñ�Һ��ƽ�����ΪV=![]() mL=17.0mL�������c(��)=
mL=17.0mL�������c(��)=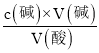 =
=![]() =0.425mol/L��
=0.425mol/L��


 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����4 mol A�����4 mol B�����Ϸ���2 L�ĺ����ܱ������У���һ�������·�����Ӧ��2A(g)+2B(g)![]() C(g)+2D(g)����5min�ﵽƽ�⣬ �����ϵ��C���������Ϊ1/9 ������˵������ȷ����( )
C(g)+2D(g)����5min�ﵽƽ�⣬ �����ϵ��C���������Ϊ1/9 ������˵������ȷ����( )
A.D��ƽ������Ϊ0.32mol/(L��min)
B.B��ת����Ϊ40%
C.����B��B��ƽ��ת��������
D.�������ϵ��ѹǿ��ƽ�������ƶ�����ѧƽ�ⳣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л��������˵����ȷ����
A. ��ȥ�Ҵ��е�����ˮ�������Ǽ���������ʯ�ң������˺��Ҵ�
B. HOCH2CH(CH3)2��(CH3)3COH����̼���칹
C. ��ȥ���������е�������Ҵ����ʣ��ɼ��������ռ���Һ��ͨ����Һ������������
D. һ���������Ѿ�����-CH3��-CH2CH3��-OH���ֻ��ţ�����ڱ�����������һ��-CH3����ͬ���칹����16��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̽��![]() ��Ӧ������Ӱ�����أ����������ʵ�顣����˵����ȷ����
��Ӧ������Ӱ�����أ����������ʵ�顣����˵����ȷ����
��ƿ��� |
| ����ˮ |
| ��Ӧ�¶� | ���dz���ʱ�� | ��ע |
1 |
| 0 mL | 10 mL |
| 10s | |
2 | 10mL | 5mL | 5 mL |
| 16 s | |
3 | 10mL | 0mL | 10 mL |
| 5 s | ��10s��ʼ���Dz������� |
4 | 10mL | 4mL |
| 8s |
A.�÷�ӦҲ��ͨ����![]() ������仯����ʾ��ѧ��Ӧ���ʵĿ���
������仯����ʾ��ѧ��Ӧ���ʵĿ���
B.3��ƿ��![]() ����ʾ����Ϊ
����ʾ����Ϊ![]()
C.��2��ƿ��3��ƿʵ�����ɵ��¶�Խ�߷�Ӧ����Խ��
D.��1��ƿ��4��ƿʵ�����ɵ��¶�Խ�߷�Ӧ����Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ļ������ڻ�����������Ҫ�����ã�
(1)����������![]() mol������
mol������![]() mol�����ں��¡��ݻ��㶨Ϊ1L���ܱ������з�Ӧ���ɰ�����20min��ﵽƽ�⣬���������ʵ���Ϊ
mol�����ں��¡��ݻ��㶨Ϊ1L���ܱ������з�Ӧ���ɰ�����20min��ﵽƽ�⣬���������ʵ���Ϊ![]() mol��
mol��
�ٸ������µ�����ת������______�����¶���![]() �Ļ�ѧƽ�ⳣ����______
�Ļ�ѧƽ�ⳣ����______![]() ����С�������λ
����С�������λ![]() ��
��
���ڵ�25minʱ�������¶Ȳ��䣬���������Ѹ��������2L�����ֺ��ݣ���ϵ�ﵽƽ��ʱ![]() ����ת����Ϊ
����ת����Ϊ![]() ������ת���ʼ�С��ԭ����______��
������ת���ʼ�С��ԭ����______��
�ۺϳɰ���Ӧ��![]()
![]() ���ڷ�Ӧ������ֻ�ı�һ������������Ӧ���ʵı仯��ͼ��ʾ��
���ڷ�Ӧ������ֻ�ı�һ������������Ӧ���ʵı仯��ͼ��ʾ��![]() ʱ�ı��������______��
ʱ�ı��������______��![]() ʱ�ı��������______��
ʱ�ı��������______��
(2)��![]() ��һ�ָ���ȼ�ϣ���ǿ��ԭ�ԣ���ͨ��
��һ�ָ���ȼ�ϣ���ǿ��ԭ�ԣ���ͨ��![]() ��NaClO��Ӧ�Ƶã�д�����Ʊ���Ӧ�Ļ�ѧ����ʽ______��
��NaClO��Ӧ�Ƶã�д�����Ʊ���Ӧ�Ļ�ѧ����ʽ______��
��N2H4��ˮ��Һ�������ԣ�����������볣��![]() ����
����![]()
![]() ˮ��Һ��pH����______
ˮ��Һ��pH����______![]() ����
����![]() �Ķ��������
�Ķ��������![]() �ĵ���
�ĵ���![]() ��
��
����֪298K��101kPa�����£�
![]()
![]()
![]()
![]()
��![]() ��ȼ����
��ȼ����![]() ______��
______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
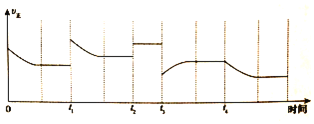
����Ŀ��һ���¶��£����ݻ�Ϊ2 L���ܱ�������ͨ���������壬������ѧ��Ӧ���������������壬��Ӧ�и����ʵ����ʵ����仯��ͼ��ʾ���Ը÷�Ӧ���ƶϺ�������
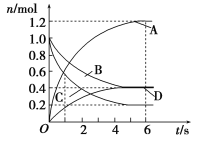
A. �÷�Ӧ�Ļ�ѧ����ʽΪ3B(g)��4D(g)![]() 6A(g)��2C(g)
6A(g)��2C(g)
B. ��Ӧ���е�1 sʱ��v(A)��v(D)
C. ��Ӧ���е�6 sʱ��B��ƽ����Ӧ����Ϊ0.05 mol/(L��s)
D. ��Ӧ���е�6 sʱ�������ʵķ�Ӧ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й��Ȼ�ѧ����ʽ����������ȷ����
A.1mol����ȼ��������̬ˮ�Ͷ�����̼���ų��������Ǽ����ȼ����
B.��N2O4(g)![]() 2NO2(g)��H=" -56.9" kJ��mol-1����֪��1mol N2O4(g)�����ܱ������г�ַ�Ӧ��ų�����Ϊ56.9kJ
2NO2(g)��H=" -56.9" kJ��mol-1����֪��1mol N2O4(g)�����ܱ������г�ַ�Ӧ��ų�����Ϊ56.9kJ
C.�ɣ�H+(aq)��OH��(aq)��H2O(l)��H����57.3kJ/mol����֪����1mol CH3COOH����Һ�뺬1mol NaOH����Һ��ϣ��ų�����Ϊ57.3 kJ
D.��֪101kPaʱ��2C(s)��O2(g)===2CO(g) ��H����221kJ/mol����1 mol̼��ȫȼ�շų�����������110.5kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£�10 mL 0.40 mol/L H2O2��Һ�������ֽ⡣��ͬʱ�̲������O2�����(������Ϊ��״��)���±���
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
������������ȷ����(��Һ����仯���Բ���)( )
A.0��6 min��ƽ����Ӧ���ʣ�v(H2O2)��3.3��10��2mol��L��1��min��1
B.0��4min��ƽ����Ӧ���ʣ�v(H2O2)>3.3��10��2mol��L��1��min��1
C.��Ӧ��6 minʱ��H2O2�ֽ���50%
D.��Ӧ��6 minʱ��c(H2O2)��0.25 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij�¶�ʱ,��nmol/L�İ�ˮ����10mL0.1mol/L������,��ҺpH���¶�����백ˮ����仯������ͼ��ʾ�������й�˵����ȷ����
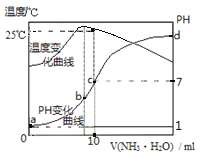
A.a��KW=1.0��10-14
B.ˮ�ĵ���̶ȣ�b>c>a>d
C.b�㣺c(NH4+)>c(Cl-)>c(H+)>c(OH-)
D.25��ʱ��һˮ�ϰ��ĵ���ƽ�ⳣ��Ϊ10-7/��10n-1������n��ʾ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com