
ijͬѧΪ�˲ⶨ�����谷�ķ���ʽ�ͽṹ��ʽ���������ʵ�顣���������ϵ�֪�������谷����Է�������Ϊ126�������谷�ڳ�����Ϊ���壬�ڼ�����������������������Ӧ���ɶ�����̼��������ˮ������12.6 g�����谷���尴��ͼ��ʾʵ��װ�÷�Ӧ(���������谷��ȫת���ɲ���)��
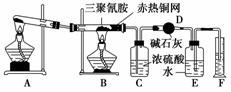
(1)д��Aװ���з�����Ӧ�Ļ�ѧ����ʽ��______________________________________________��
(2)Cװ���ܲ�����Dװ�û�����________(��ܡ����ܡ�)��������________________________________________��
(3)��Bװ���з�Ӧ��ȫ��ȡF��ˮ�������ʵ�����˳��Ϊ________(�����)��
�ٶ���������ȴ�����¡��۵�ƽE��Fװ����Һ��
(4)�ⶨ�������£�
| ���� | C | D |
| ʵ��ǰ | 101.0 g | 56.0 g |
| ʵ��� | 106.4 g | 69.2 g |
���ⶨ���ռ����������ۺϳɱ�״���µ����Ϊ6.72 L��
����������ʵ�����ݣ�ͨ�������֪�����谷��ʵ��ʽΪ________��
�������谷�ķ���ʽΪ________��
����װ����û��ͭ������Բⶨ�����Ӱ����_______________________________________________��
(5)��֪����(HCN)�Ľṹ��ʽΪH��C��N���谷�Ľṹ��ʽΪH2N��C��N�������谷������ÿ��ԭ�ӵ�������������Ϊ8��2������ṹ��ʽΪ________��
����������(1)����ʵ��ԭ��֪��Aװ��������ȡ����������Ҫ���ȡ�(2)��ʯ�Ҽ������ն�����̼��������ˮ����Ũ����ֻ����ˮ���������ն�����̼��(3)E���ռ��������¶Ⱥ�ѹǿ��ֱ�Ӳⶨ��ֻ�ܲⶨʵ�������������¶Ⱥ�ѹǿ�����ԣ�����ǰӦ����ȴװ�ã�ʹE�������¶��������ͬ��Ȼ���ƽE��F�е�Һ�档
(4)Cװ�þ���������������ˮ��������Dװ�þ��������������ɶ�����̼����������n(CO2)��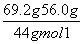 ��0.3 mol��n(H2O)��
��0.3 mol��n(H2O)�� ��0.3 mol��n(N2)��
��0.3 mol��n(N2)�� ��0.3 mol��m(O)��12.6 g��(0.3 mol��12 g��mol��1��0.6 mol��1 g��mol��1��0.6 mol��14 g��mol��1)��0��˵����Ʒ�в�����Ԫ�ء��ʷ����У�n(C)��n(H)��n(N)��0.3 mol��0.6 mol��0.6 mol��1��2��2��
��0.3 mol��m(O)��12.6 g��(0.3 mol��12 g��mol��1��0.6 mol��1 g��mol��1��0.6 mol��14 g��mol��1)��0��˵����Ʒ�в�����Ԫ�ء��ʷ����У�n(C)��n(H)��n(N)��0.3 mol��0.6 mol��0.6 mol��1��2��2��
�������谷�ķ���ʽΪ(CH2N2)n������(12��2��28)��n��126�����n��3���ʷ���ʽΪC3H6N6����û��ͭ�����������δ���뷴Ӧ����������Eƿ�����²ⶨ���������ƫ���ⶨ�������谷�ķ���ʽ�е�ԭ����ƫ��̼ԭ��������ԭ����ƫС��
(5)��������谷�ķ���ʽC3H6N6��֪��H2N��C��N�ӳ�������Ԫ��״�����谷��
���𰸡���(1)2KMnO4 K2MnO4��MnO2��O2����2KClO3
K2MnO4��MnO2��O2����2KClO3 2KCl��3O2��
2KCl��3O2��
(2)���ܡ�Ũ��������ˮ����ʯ�����ն�����̼�������������ʯ����ͬʱ���ն�����̼��ˮ�����ֱܷ���ˮ�������̼���������Ӷ�����ʵ��ʧ��
(3)�ڢۢ�
(4)��CH2N2����C3H6N6���۲ⶨ���÷����е�ԭ����ƫ��̼����ԭ����ƫС



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�ijˮ��ҺM�д��ڵ������У�Na����A2����HA����H����OH�������ڵķ�����H2O��H2A����������ش��������⣺
(1)д����H2A�ĵ��뷽��ʽ______________________________________________
________________________________________________________________________��
(2)����ҺM��10 mL 2 mol��L��1NaHA��Һ��10 mL 2 mol��L��1 NaOH��Һ��϶��ã�����ҺM��pH____________7(�����������������)����Һ������Ũ���ɴ�С��˳��Ϊ________________________________________________________________________��
��֪Ksp(BaA)��1.8��10��10����û����Һ�м���10 mL 1 mol��L��1BaCl2��Һ����Ϻ���Һ�е�Ba2��Ũ��Ϊ________ mol��L��1��(���Ի�Ϻ���Һ����ı仯)
(3)����ҺM�����������������0.01 mol��L��1��H2A��Һ����0.01 mol��L��1��NaHA��Һ����0.02 mol��L��1��HCl��0.04 mol��L��1��NaHA��Һ��������Һ���������������Һ��H2A����Ũ������Ϊ____________��pH�ɴ�С��˳��Ϊ____________��(����д���)
(4)����ҺM��pH��3��H2A��ҺV1 mL��pH��11��NaOH��ҺV2 mL��Ϸ�Ӧ���ã������Һc(H��)/c(OH��)��104��V1��V2�Ĵ�С��ϵΪ________(����ڡ������ڡ���С�ڡ����п��ܡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� �й��ڻ�ʯȼ�ϵļӹ�˵����ȷ����
�й��ڻ�ʯȼ�ϵļӹ�˵����ȷ����
A��ʯ���ѻ���Ҫ�õ���ϩ
B��ʯ�ͷ����ǻ�ѧ�仯���ɵõ����͡�ú��
C��ú������Ҫ�õ���̿��ú���͡��ְ�ˮ�ͽ�¯��
D��ú��ú���������仯���Ǹ�Ч����������ú����Ҫ; ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ�п��ܺ���Na����NH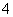 ��Ba2����SO
��Ba2����SO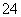 ��I����S2�����ֱ�ȡ��������pH�Ʋ��ԣ���Һ�������ԣ��ڼ���ˮ�͵�������������Ϊȷ����Һ����ɣ���������������(����)
��I����S2�����ֱ�ȡ��������pH�Ʋ��ԣ���Һ�������ԣ��ڼ���ˮ�͵�������������Ϊȷ����Һ����ɣ���������������(����)
A��Na��������B��SO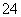 ������C��Ba2��������D��NH
������C��Ba2��������D��NH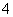
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���������ܴﵽĿ�ĵ���(����)
A������ˮ���𱽡��Ҵ������Ȼ�̼
B����������ͭ��ĩ��������
C����ʯ����Һ����������Ҵ�
D���ü���ʼ��������Һ�Ķ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵��û�п�ѧ�Դ������( )
A������ѩ�ơ�Ư������������ɫ������Ȼʧ��ɫ����û����ף�ֻ�и���
B��Һ̬�Ȼ�����100%�����ᣬH+Ũ�ȼ���
C���Ȼ��ƾ�����ˮ�Ժͳ����ԣ�������������ˮ��Һ��������·������Ч�ر�������
D����Ԫ���ж����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��ַ����У��������ڳ�ȥ�մ��л�������С�մ���ǣ� ��
A��ˮϴ B������ C��ͨ��CO2 D����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������棬��2011��5��1���𣬽�ֹ��������������ӹ������ƣ�CaO2����ʳƷ���Ӽ������ж��ڹ�������(CaO2)������������ǣ� ��
A��CaO2���������ԣ�����ۿ��ܾ�����������
B��CaO2���������ӵĸ�����Ϊ1��1
C��CaO2��ˮ��Ӧʱ��ÿ����1 mol O2ת�Ƶ���4 mol
D��CaO2��CO2��Ӧ�Ļ�ѧ����ʽΪ��2CaO2 +2CO2 ��2CaCO3+O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���и�����Һ��Ϻ�����Һ��ǡ�ó����Ե����ӷ���ʽ�� �� ��
��Ba(OH)2��NaHSO4 ��Ba2+ +2OH�� +2H+ +SO42����BaSO4��+ 2H2O
��Ba(OH)2��NaHSO4 ��Ba2+ + OH��+ H++ SO42����BaSO4��+ H2O
�� Ba(OH)2��KAl(SO4)2��2Ba2+ + 4OH��+ Al3++ 2SO42����2BaSO4��+ AlO2��
��Ba(OH)2��KAl(SO4)2��3Ba2+ + 6OH��+ 2Al3++ 3SO42����3BaSO4��+2Al(OH)3��
A���ۢ� B���ڢ� C���٢� D���٢�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com