
 CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺

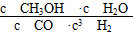 ���ڴ��ڡ���a��b��c
���ڴ��ڡ���a��b��c

 ȫ��������ϵ�д�
ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ������Ӧ�Ļ�ѧ��ӦΪ��__ ___
������Ӧ�Ļ�ѧ��ӦΪ��__ ___| ���� | �ݻ�/L | �¶�/�� | ��ʼ��/mol | ƽ����/mol[ | �ﵽƽ������ʱ��/min | |
| C(s) | H2O(g) | H2(g) | ||||
| �� | 2 | T1 | 2 | 4 | 3.2 | 8 |
| �� | 1 | T2 | 1 | 2 | 1,2 | 3 |
 CH3OCH3(g��+ 3H2O(g)
CH3OCH3(g��+ 3H2O(g)| Ͷ�ϱ�[n(H2��/ n(CO2)] | 500 K | 600 K | 700 K | 800 K |
| 1.5 | 45% | 33% | 20% | 12% |
| 2.0 | 60% | 43% | 28% | 15% |
| 3.0 | 83% | 62% | 37% | 22% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 Fe3+(aq)+3OH-(aq) �� ��H=" a" kJ?mol-1
Fe3+(aq)+3OH-(aq) �� ��H=" a" kJ?mol-1 H+(aq)+OH-(aq) ����H=" b" kJ?mol-1
H+(aq)+OH-(aq) ����H=" b" kJ?mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
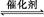 2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��


 N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol
N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol N2O4(g) ��H2="-56.9" kJ/mol
N2O4(g) ��H2="-56.9" kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 TiCl4(l)��H="-804.2" kJ/mol
TiCl4(l)��H="-804.2" kJ/mol 2CO2(g)�Ħ�H= kJ/mol��
2CO2(g)�Ħ�H= kJ/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
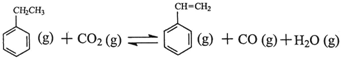 ��H
��H ��H1=��117.6kJ��mol��1
��H1=��117.6kJ��mol��1 CO (g)��H2O (g) ��H2=��41.2kJ��mol��1
CO (g)��H2O (g) ��H2=��41.2kJ��mol��1 ��
��| A������0.5mol/L | B����0.5mol/L |
| C������0.5mol/L | D����ȷ�� |
 ����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú�
����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú� ��P�ı���ʽ��ʾ����
��P�ı���ʽ��ʾ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| ʵ���� | T(��) | n(N2)/n(H2) | p(MPa) |
| �� | 450 | 1/3 | 1 |
| �� | �� | �� | 10 |
| �� | 480 | �� | 10 |
 2NH3(g)���ص�,�ڸ���������ͼ2��,��������1 MPa��10 MPa������H2��ת�������¶ȱ仯����������ʾ��ͼ,�������������ߵ�ѹǿ��
2NH3(g)���ص�,�ڸ���������ͼ2��,��������1 MPa��10 MPa������H2��ת�������¶ȱ仯����������ʾ��ͼ,�������������ߵ�ѹǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com