
����Ŀ��ʹ���ʸ������������(Na2SiF6)Ϊԭ�Ϻϳɱ���ʯ(Na3AlF6)��Ϊһ������������Դ����߾���Ч����·�����������������ͼ��ʾ��
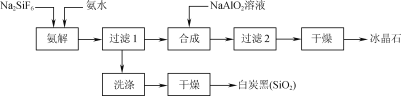
��1����ͳ�ϳɱ���ʯ�ķ�����өʯ(CaF2)����ʹ��өʯ��ʯӢ�ʹ����ڸ�������������NaF����NaF��Һ�м���Al2(SO4)3��Һ�Ƶá��ڼ�����������Һǰ�����������ὫNaF��Һ��pH�µ���5���ң�
������ܲ���������____(�����ʵĻ�ѧʽ)������Ȳ��˹�ǿ��ԭ����____��
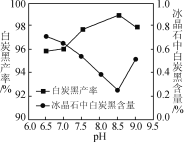
��2������ʱ��̿�ڲ��ʺͱ���ʯ������pH�Ĺ�ϵ��ͼ������ʱ��Ҫ������Һ��pH��____������߰������ʵĴ�ʩ��____(����ĸ)��
A�����ٽ���
B�����Ȼ��Һ��100��
C����С��ˮŨ��
��3�����������а��ⷴӦ�Ļ�ѧ����ʽΪ____������ʯ�ϳɷ�Ӧ�����ӷ���ʽΪ______��
��4��Ϊ�����ԭ�������ʣ����ٻ�����Ⱦ���ɲ�ȡ�Ĵ�ʩ��___��
���𰸡�Al(OH)3 H����F�������������HF��ʹF��Ũ�ȼ��Ͳ�������Na3AlF6 8.5 A Na2SiF6��4NH3��H2O=2NaF��4NH4F��SiO2����2H2O 3Na����4NH4+��6F����AlO2-��2H2O=Na3AlF6����4NH3��H2O ����2����Һ��ˮ��ѭ������
��������
��������ͼ����ˮ��ȡ��������ʱ�����˶������裬��ͬʱ������NaF��NH4F��H2O����Ӧ�ķ���ʽ���Ա�ʾΪNa2SiF6+4NH3H2O�T2NaF+4NH4F+ SiO2��+2H2O�������Һ�к���NaF��NH4F������NaAlO2��Һ�õ�Na3AlF6������2�õ�����ҺΪ����ˮ��Һ����ѭ��ʹ�ã��ݴ˷������
(1)��ͳ�ϳɱ���ʯ�ķ�����өʯ(CaF2)������ʹ��өʯ��ʯӢ�ʹ����ڸ�������������NaF����NaF��Һ�м���Al2(SO4)3��Һ�Ƶá�NaFˮ�⣬��Һ�Լ��ԣ��ڼ�����������Һǰ�����������ὫNaF��Һ��pH�µ���5���ң���ֹ����������������Al(OH)3����������Ȳ��˹�ǿ������H����F�������������HF��ʹF��Ũ�ȼ��Ͳ�������Na3AlF6���ʴ�Ϊ��Al(OH)3��H����F�������������HF��ʹF��Ũ�ȼ��Ͳ�������Na3AlF6��
(2)��ͼ��֪��pHΪ8.5ʱ������ʯ�а�̿�ڵĺ����ϵͣ�����ʯ���Ƚϸߣ���̿�ڲ��ʸߣ�A�����ٽ�����Լӿ췴Ӧ���ʣ���A��ȷ��B�����Ȼ��Һ��100������ˮ���ȷֽ⣬��Ӧ��Ũ��С����ѧ��Ӧ���ʼ�������B����C����С��ˮŨ�ȣ���ѧ��Ӧ���ʼ�������C���ʴ�Ϊ��8.5��A��
(3) ��������ͼ����ˮ��ȡ��������ʱ�����˶������裬��ͬʱ������NaF��NH4F��H2O����Ӧ�ķ���ʽ���Ա�ʾΪNa2SiF6+4NH3H2O�T2NaF+4NH4F+ SiO2��+2H2O��ˮԡ���ȹ��������ɱ���ʯ�Ļ�ѧ����ʽΪ��2NaF+4NH4F+NaAlO2+2H2O= Na3AlF6��+4NH3��H2O�����ӷ���ʽΪ3Na��+4NH4++6F��+AlO2-+2H2O=Na3AlF6��+4NH3��H2O���ʴ�Ϊ��Na2SiF6+4NH3H2O�T2NaF+4NH4F+ SiO2��+2H2O��3Na��+4NH4++6F��+AlO2-+2H2O=Na3AlF6��+4NH3��H2O��
(4)���������������ڶ��ι���������ҺΪ��ˮ��Һ��Ϊ�����ԭ�������ʣ����ٻ�����Ⱦ����ѭ��ʹ�ð�ˮ���ʴ�Ϊ������2����Һ��ˮ��ѭ�����á�


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������(F)��һ�����������ҩ����������ϵͳ�����Ե��˷ܺ�����˫�����ã���ϳ�·������ͼ��ʾ����ش�������⡣
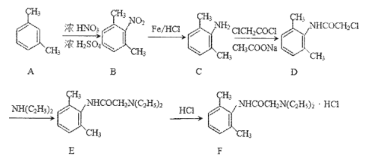
��1��A��ϵͳ������_____��D�еĹ�����������___��
��2��F�Ļ�ѧʽ��_____��
��3��B��C��D��E�ķ�Ӧ����������_____��_____��
��4��D������NaOH��Һ���ȳ�ַ�Ӧ�ķ���ʽ��_____��
��5��G�����DZ�B��һ��̼ԭ�ӵ�ͬϵ���������Ҫ���G��ͬϵ����___�֣������������칹�������к˴Ź���������������������Ϊ3:2:2:2:1:1�Ľṹ��ʽ��____(��дһ��)��______
I.������������ȡ����������һ������̼ԭ�� II.����һCOO���ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ʊ�N2H4��H2O��ˮ���£�����ˮNa2SO3��Ҫʵ���������£�

��֪���� �������ռ���Һ�ķ�Ӧ�Ƿ��ȷ�Ӧ��
�� N2H4��H2O��ǿ��ԭ�ԣ�����NaClO���ҷ�Ӧ����N2��
�Ŵ����̷��������������õ���Ҫ�л�ԭ��Ϊ_______________��д���ƣ���
�Ʋ������Ʊ�NaClO��Һʱ�����¶�Ϊ41������ò����г�NaClO�����NaClO3�����������ʵ���֮��Ϊ5��1���÷�Ӧ�����ӷ���ʽΪ____________________��
��ʵ���У�Ϊʹ�������з�Ӧ�¶Ȳ�����40 ����������Cl2��ͨ�������⣬���ɲ�ȡ�Ĵ�ʩ��_________________��
�Ȳ������ϳ�N2H4��H2O���е�Լ118 ������װ����ͼ��NaClO������Һ������[CO��NH2��2]���е�196.6����ˮ��Һ��40�����·�Ӧһ��ʱ�����Ѹ��������110��������Ӧ��

�� ʹ�������ܵ�Ŀ����_________________��
�� ��Һ©���ڵ��Լ���_______��
����Һ©���ڵ�Һ�����������ƿ�ڵIJ�����______________________________��
�� д������������ˮ���·�Ӧ�Ļ�ѧ����ʽ________________________________��
�� �������Ʊ���ˮNa2SO3��ˮ��Һ��H2SO3��HSO3����SO32����pH�ķֲ���ͼ��ʾ����

�� �߽������Na2CO3��Һ��ͨ��SO2�Ʊ�NaHSO3��Һ��ʵ����ȷ��ֹͣͨSO2��pHֵΪ____��ȡ��������ֵ����ͬ����
�����Ƶõ�NaHSO3��Һ����Na2SO3��Һ��pHӦ������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ں��¡����Ϊ2 L���ܱ������н��з�Ӧ��2A(g) ![]() 3B(g)��C(g)������Ӧ����ǰ20 s��3 mol��Ϊ1.8 mol����ǰ20 s��ƽ����Ӧ����Ϊ
3B(g)��C(g)������Ӧ����ǰ20 s��3 mol��Ϊ1.8 mol����ǰ20 s��ƽ����Ӧ����Ϊ
A. v(B)��0.03 mol��L��1��s��1 B. v(B)��0.045 mol��L��1��s��1
C. v(C)��0.03 mol��L��1��s��1 D. v(C)��0.06 mol��L��1��s��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����84����Һ����1984�걱��ijҽԺ����ʹ�ö����������ճ�������ʹ�ù㷺������Ч�ɷ���NaClO��ij��ѧ�о���ѧϰС����ʵ�����Ʊ�NaClO��Һ������������̽���ͳɷֲⶨ��

(1)��ѧϰС�鰴��ͼװ�ý���ʵ�飨���ּг�װ��ʡȥ������Ӧһ��ʱ��ֱ�ȡB��Cƿ�е���Һ����ʵ�飬ʵ���������±���
��֪���ٱ���NaClO��ҺpHΪ11��
��25��Cʱ��������볣��Ϊ��H2CO3��K1=4.4��10-7��K2=4.7��10-11��HClO��K=3��10-8
ʵ�鲽�� | ʵ������ | |
Bƿ | Cƿ | |
ʵ��1��ȡ�����μ���ɫʯ����Һ | ��죬����ɫ | ����������ɫ |
ʵ��2���ⶨ��Һ��pH | 3 | 12 |
�ش��������⣺
������a������___________��װ��A�з�����Ӧ�����ӷ���ʽ__________��
��Cƿ��Һ�е�������NaCl��__________���ѧʽ����
������Cƿ��Һ����NaHCO3��Һ�������������������ʵ�飬Cƿ����Ϊ��ʵ��1����ɫʯ����Һ������ɫ��ʵ��2����Һ��pH=7�����ƽ���ƶ�ԭ��������ɫʯ����Һ������ɫ��ԭ��______
(2)�ⶨCƿ��Һ��NaClO��������λ��g/L����ʵ�鲽�����£�
��ȡCƿ��Һ20mL����ƿ�У����������ữ���������KI��Һ���ǽ�ƿ�����ڰ�����ַ�Ӧ��
����0.1000mol/LNa2S2O3����Һ�ζ���ƿ�е���Һ��������Һ��ʾ�յ���ظ�����2��3�Σ�Na2S2O3��Һ��ƽ������Ϊ24.00mL������֪��I2+2S2O32-=2I-+S4O62-��
�ٲ���I��Cƿ�з�����Ӧ�����ӷ���ʽΪ_________��
�ڸǽ�ƿ�����ڰ�����Ӧ��ԭ��__________�ζ����յ������_____________��
��Cƿ��Һ��NaClO����Ϊ______g/L������2λС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ���ǣ� ��
A. ����������Һ�м�������泥�Ba2����OH����NH4+��SO42-=BaSO4����NH3��H2O
B. �ö��Ե缫���CuCl2��Һ��Cu2����2Cl����2H2O![]() Cu(OH)2����H2����Cl2��
Cu(OH)2����H2����Cl2��
C. ��Ư����Һ��ͨ��������������Ca2����2ClO����SO2��H2O=CaSO3����2HClO
D. ������Һ��ͨ��������CO2��C6H5O����CO2��H2O��C6H5OH��HCO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A����һ��ֲ���������ڼ���A�ɷ�����ͼ��ʾ��һϵ�л�ѧ��Ӧ��

��ͼ�ش��������⣺
(1)д��A��C��D�Ľṹ��ʽ��
A________��C________��D________��
(2)д���٢�������Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��________________________________(��Ӧ����____________)��
��________________________________(��Ӧ����____________)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
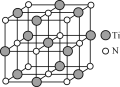
����Ŀ��������(Ti)��һ�־��������������ܵĽ������Ѻ��ѺϽ���Ϊ��21���͵���Ҫ�������ϡ�
��1��Ti(BH4)2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ�
��Ti2����̬�����Ų�ʽ�ɱ�ʾΪ_____��
����BH4-��Ϊ�ȵ�����������ӵĻ�ѧʽΪ_____��
��H��B��Tiԭ�ӵĵ�һ��������С�����˳��Ϊ_____��
��2����������(TiO2)�dz��õġ����нϸߴ����Ժ��ȶ��ԵĹ��������������ˮ����������TiO2����һ��ʵ����ͼ��ʾ��
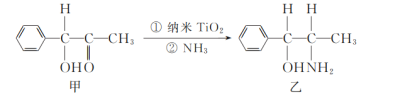
�������ҵķ����в�ȡsp3�ӻ���ʽ��ԭ�Ӹ���Ϊ_____��
��3��ˮ��Һ�в�û��[Ti(H2O)6)]4�����ӣ�����[Ti(OH)2(H2O)4]2�����ӣ�1mol[Ti(OH)2(H2O)4]2������������ĿΪ____��
��4��������(TiN)���е��͵�NaCl�ͽṹ��ij̼�����ѻ�����ṹ����̼ԭ��ȡ�������Ѿ���(�ṹ��ͼ)����ĵ�ԭ�ӣ����̼�����ѻ�����Ļ�ѧʽ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(ClNO)���л���ϳ��е���Ҫ�Լ�����е�Ϊ��5.5�棬��ˮ�⡣��֪��AgNO2����ˮ�����������ᣬAgNO2+HNO3=AgNO3 +HNO2��ijѧϰС����ʵ������Cl2��NO�Ʊ�ClNO���ⶨ�䴿�ȣ����ʵ��װ����ͼ��ʾ��
(1)�Ʊ�Cl2�ķ���װ�ÿ���ѡ��___________(����ĸ����)װ�ã�������Ӧ�����ӷ���ʽΪ___________��
(2)���ռ�һƿ�����������ѡ����ʵ�װ�ã�������˳��Ϊ a��________________(������������Сд��ĸ��ʾ)��
(3)ʵ���ҿ���ͼʾװ���Ʊ��������ȣ�
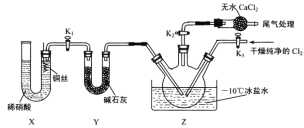
��ʵ����Ҳ���� B װ���Ʊ� NO �� Xװ�õ��ŵ�Ϊ___________________________________��
�ڼ���װ�������Բ�װ��ҩƷ����K2��Ȼ���ٴ�K3��ͨ��һ��ʱ�����壬��Ŀ����____________��Ȼ�����������������Z����һ����Һ������ʱ��ֹͣʵ�顣
(4)��֪��ClNO ��H2O��Ӧ����HNO2�� HCl��
�����ʵ��֤�� HNO2�����_____________��(���ṩ���Լ���1 molL-1���ᡢ 1 molL-1HNO2��Һ�� NaNO2��Һ����ɫʯ����ֽ����ɫʯ����ֽ)��
��ͨ������ʵ��ⶨClNO��Ʒ�Ĵ��ȡ�ȡZ������Һ��m g ����ˮ�����Ƴ�250 mL ��Һ��ȡ��25.00 mL��Ʒ������ƿ�У���K2CrO4��ҺΪָʾ������c molL-1 AgNO3����Һ�ζ����յ㣬���ı���Һ�����Ϊ20.00mL���ζ��յ��������__________����������(ClNO)����������Ϊ_________��(��֪�� Ag2CrO4Ϊש��ɫ���壻 Ksp(AgCl)��1.56��10-10��Ksp(Ag2CrO4)��1��10-12)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com