������ʡ���¼�����������Ӧ�ò��Ϲ�˾��������ҵ�������ҵ�����˲�ҵ�У��趼��������Ҫ�����ã���ش��������⣺
��1������ʹ�ù����β�Ʒ���������մɵȣ�����ʷ�Ѿ���һ�����ˣ�����1823��Ż�õ��ʹ裬��仯ѧ�ұ�����˹�ý����ػ�ԭSiF
4��õ��ʹ裬д���÷�Ӧ�Ļ�ѧ����ʽ
��
��2��������ȡ�����������IJ�ͬ���õ��ĵ��ʹ���̬��ͬ��������Ҳ��ͬ��
�������ȷ���ԭ��������Ƶýϴ����ľ���裺4Al+3K
2SiF
6�T3Si+2KAlF
4+2K
2AlF
3�����ڸ÷�Ӧ������˵����ȷ����
������ţ���
A��Al�ǻ�ԭ�� B����������ֻ��KAlF
4C��ÿת��6N
A���ӣ��õ�42 g Si D��AlԪ����KAlF
4��K
2AlF
5�л��ϼ۲�ͬ
���ð�ɰ����þ�ۻ���ڸ��������µõ����ι裬�÷�Ӧ�Ļ�ѧ����ʽΪ
��
�����ι�;����Ľṹ�кܴ�IJ�����ι�Ϊ��ɫ��ĩ����������Ǵ���ɫ���������й���ĵ�����澧�壬���ι�Ļ�ѧ���ʱȾ������õö࣮ͨ�������������ܵó��Ľ�����
��дһ�����ɣ���
��3����������ʱ��������������ԭ��ұ�����������γɵ�FeO���䷴Ӧ�Ļ�ѧ����ʽΪ
��
��4����Ұ�⣬Ϊ��Ѹ�ٵõ��������ù��������Ca��OH��
2��NaOH��ϣ�����ǿ�ȣ�����Ѹ�ٵõ�H
2��NaSiO
3��CaO�����ֻ����������������д���÷�Ӧ�Ļ�ѧ����ʽ
��
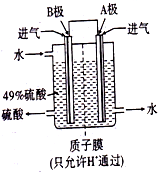
��5��ij���е�λ����ԭ���ԭ������SO
2��O
2���Ʊ����ᣬװ����ͼ��ʾ���缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���
��B�缫�ĵ缫��ӦʽΪ
��
����Һ��H
+���ƶ�������
����
����

 ������ʡ���¼�����������Ӧ�ò��Ϲ�˾��������ҵ�������ҵ�����˲�ҵ�У��趼��������Ҫ�����ã���ش��������⣺
������ʡ���¼�����������Ӧ�ò��Ϲ�˾��������ҵ�������ҵ�����˲�ҵ�У��趼��������Ҫ�����ã���ش��������⣺


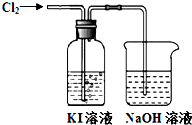 ijͬѧ��Cl2��KI��Һ�ķ�Ӧ������ʵ��̽������Ӧװ�����£�
ijͬѧ��Cl2��KI��Һ�ķ�Ӧ������ʵ��̽������Ӧװ�����£�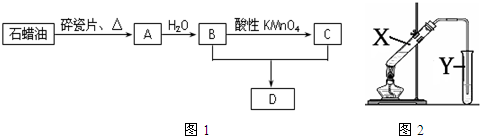

 +CH3Cl
+CH3Cl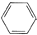 -CH3+HCl
-CH3+HCl
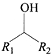 ��1R��R2�����ǻ���
��1R��R2�����ǻ���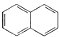 �ṹ��������FeCl3��Һ������ɫ��Ӧ������
�ṹ��������FeCl3��Һ������ɫ��Ӧ������