
،¾جâؤ؟،؟زرضھ£؛³£خآدآ£¬(1)Ka1(H2CO3)=4.3،ء10-7£¬Ka2(H2CO3)=5.6،ء10-11£»(2)H2R¼°ئنؤئرخµؤبـز؛ضذ£¬H2R،¢HR-،¢R2-·ض±ًشعبصكضذثùص¼µؤخïضتµؤء؟·ضت(¦ء)ثوبـز؛pHµؤ±ن»¯¹طدµبçح¼ثùت¾،£دآءذذًتِ´يخَµؤتا£¨ £©

A.شعpH=1.3µؤبـز؛ضذ£؛c(Na+)£¼c(H2R)+2c(R2-)
B.µبجه»µبإ¨¶بµؤNaOHبـز؛سëH2Rبـز؛»ى؛د؛َ£¬بـز؛ضذث®µؤµçہë³ج¶ب±ب´؟ث®ذ،
C.شعpH=2µؤبـز؛ضذ´وشع![]() =10-3
=10-3
D.دٍNa2CO3بـز؛ضذ¼سبë¹ء؟H2Rبـز؛£¬·¢ةْ·´س¦£؛CO32-+H2R=HCO3-+HR-
،¾´ً°¸،؟D
،¾½âخِ،؟
A£®شعpH=1.3µؤبـز؛ضذ£¬c(H2R)=c(HR-)£¬بـز؛ضذµç؛ةتط؛مخھ£؛2c(R2-)+c(HR-)+c(OH-)=c(Na+)+c(H+)£¬بـز؛دشثلذش£¬c(OH-)£¼c(H+)£¬شٍc(Na+)£¼2c(R2-)+c(HR-)=c(H2R)+2c(R2-)£¬¹تAصب·£»
B£®µبجه»µبإ¨¶بµؤNaOHبـز؛سëH2Rبـز؛»ى؛د؛َ£¬ةْ³ةNaHR£»سةح¼؟ةضھ£¬بـز؛ضذHR-،¢R2-إ¨¶بدàح¬ت±£¬pH=4.3£¬بـز؛دشثلذش£¬ثµأ÷HR-µؤµçہë×÷سأ½دا؟£¬¶شث®µؤµçہëئًµ½زضضئ×÷سأ£¬ثùزشبـز؛ضذث®µؤµçہë³ج¶ب±ب´؟ث®ذ،£¬¹تBصب·£»
C£®µ±بـز؛pH=1.3ت±£¬c(H2R)=c(HR-)£¬شٍKa1= 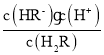 =10-1.3£¬بـز؛µؤpH=4.3ت±£¬c(R2-)=c(HR-)£¬شٍKa2=
=10-1.3£¬بـز؛µؤpH=4.3ت±£¬c(R2-)=c(HR-)£¬شٍKa2=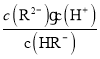 =10-4.3£¬شعpH=2µؤبـز؛ضذ´وشع
=10-4.3£¬شعpH=2µؤبـز؛ضذ´وشع![]() =
=![]() =10-3£¬¹تCصب·£»
=10-3£¬¹تCصب·£»
D£®سةCضذ·ضخِضھH2Rµؤµçہë³£تKa2´َسعH2CO3µؤKa1£¬¼´ثلذش£؛HR-£¾H2CO3£¬ثùزشدٍNa2CO3بـز؛ضذ¼سبë¹ء؟H2Rبـز؛£¬·¢ةْ·´س¦£؛CO32-+H2R=H2O+CO2،ü+R2-£¬¹تD´يخَ£»
¹ت´ً°¸خھD،£



| ؤ꼶 | ¸كضذ؟خ³ج | ؤ꼶 | ³ُضذ؟خ³ج |
| ¸كز» | ¸كز»أâ·ر؟خ³جحئ¼ِ£، | ³ُز» | ³ُز»أâ·ر؟خ³جحئ¼ِ£، |
| ¸ك¶ | ¸ك¶أâ·ر؟خ³جحئ¼ِ£، | ³ُ¶ | ³ُ¶أâ·ر؟خ³جحئ¼ِ£، |
| ¸كب | ¸كبأâ·ر؟خ³جحئ¼ِ£، | ³ُب | ³ُبأâ·ر؟خ³جحئ¼ِ£، |
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟جْ؛ى(Fe2O3)؛حîر°×·غ(TiO2)¾ùخھضطزھµؤا½أو×°ذقرصءد،£ز»ضضہûسأîرجْ؟َ(ض÷زھ³ة·ضخھFeTiO3£¬»¹؛¬سذةظء؟Fe2O3)ءھ؛دةْ²ْجْ؛ى؛حîر°×·غµؤ¹¤زصء÷³جبçح¼ثùت¾£؛
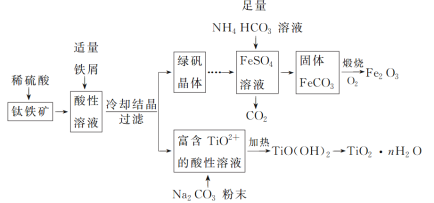
»ط´ًدآءذختجâ£؛
£¨1£©FeTiO3ضذFeµؤ»¯؛د¼غخھ_______،£
£¨2£©خھ¼س؟ىîرجْ؟َشعد،ءٍثلضذµؤبـ½â£¬؟ة²ةب،µؤ´ëت©سذ_______ (بخذ´ء½ضض)،£ثلذشبـز؛ضذ¼سبëتتء؟جْذ¼µؤؤ؟µؤتا½«_______،£
£¨3£©FeSO4بـز؛سëNH4HCO3بـز؛µؤ·´س¦خآ¶بس¦؟طضئشع35 ،وزشدآ£¬ئنشزٍتا_______£¬¸أ·´س¦µؤہë×س·½³جت½تاFe2£«£«2HCO3-=FeCO3،£«CO2،ü£«H2O،£
£¨4£©TiO2£«×ھ»¯خھTiO(OH)2ذèزھ¼سبب£¬¼سببµؤؤ؟µؤتا_______£¬¸أ·´س¦µؤہë×س·½³جت½خھTiO2£«£«2H2O![]() TiO(OH)2،£«2H£«،£
TiO(OH)2،£«2H£«،£
£¨5£©³£خآت±£¬شعةْ³ةµؤFeCO3´ïµ½³ءµيبـ½âئ½؛âµؤبـز؛ضذ£¬²âµأبـز؛ضذc(CO32-)£½3.0،ء10£6 mol،¤L£1£¬pHخھ8.5£¬شٍثùµأµؤFeCO3ضذتا·ٌ؛¬Fe(OH)2_______£؟
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟¹ْ¼تةç»ل·¢³ِآنتµ،¶°حہèذ¶¨،·£¬حئ¶¯آجة«µحج¼×ھذح£¬¹¹½¨بثہàأüشث¹²ح¬جهµؤ»¼«ذإ؛إ،£ةْج¬¹¤زµ؛حر»·¾¼أ³ةخھ×غ؛د½â¾ِبثہà×تش´،¢»·¾³؛ح¾¼أ·¢ص¹µؤز»جُسذذ§ح¾¾¶،£
£¨1£©ث®تا،°ةْأüض®»ùضت،±£¬تا،°سہش¶ضµµأج½¾؟µؤخïضت،±،£
زش²¬رô¼«؛حت¯ؤ«زُ¼«ةè¼ئµç½â³ط£¬ح¨¹µç½âNH4HSO4بـز؛²ْةْ(NH4)2S2O8£¬شظسëث®·´س¦µأµ½H2O2£¬ئنضذةْ³ةµؤNH4HSO4؟ةزشر»·ت¹سأ،£
¢ظرô¼«µؤµç¼«·´س¦ت½تا__،£
¢عضئ±¸H2O2µؤ×ـ·´س¦·½³جت½تا__،£
£¨2£©CO2µؤ×تش´»¯ہûسأؤـسذذ§¼ُةظCO2إإ·إ£¬³ن·ضہûسأج¼×تش´،£
CO2´ك»¯¼ساâ؛د³ة¶¼×أرتاز»ضضCO2×ھ»¯·½·¨£¬ئن¹³جضذض÷زھ·¢ةْدآءذ·´س¦£؛
·´س¦¢ٌ£؛CO2(g)+H2(g)=CO(g)+H2O(g) ¦¤H=41.2kJ،¤mol1
·´س¦¢ٍ£؛2CO2(g)+6H2(g)=CH3OCH3(g)+3H2O(g) ¦¤H=-122.5kJ،¤mol1
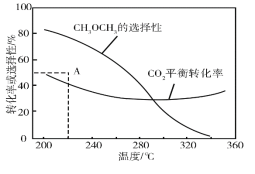
شع؛مر¹،¢CO2؛حH2µؤئًت¼ء؟ز»¶¨µؤجُ¼دآ£¬CO2ئ½؛â×ھ»¯آت؛حئ½؛âت±CH3OCH3µؤر،شٌذشثوخآ¶بµؤ±ن»¯بçح¼،£ئنضذ£؛
CH3OCH3µؤر،شٌذش=![]() ،ء100£¥
،ء100£¥
¢ظخآ¶ب¸كسع300،و£¬CO2ئ½؛â×ھ»¯آتثوخآ¶بة¸ك¶ّةدةµؤشزٍتا__،£
¢ع220،وت±£¬شع´ك»¯¼ء×÷سأدآCO2سëH2·´س¦ز»¶خت±¼ن؛َ£¬²âµأCH3OCH3µؤر،شٌذشخھ48%£¨ح¼ضذAµم£©،£²»¸ؤ±ن·´س¦ت±¼ن؛حخآ¶ب£¬ز»¶¨ؤـجل¸كCH3OCH3ر،شٌذشµؤ´ëت©سذ__،£
·دخïشظہûسأ،£بçح¼×°ضأ¼سزش±طزھµؤµ¼دكء¬½س؛َ´ïµ½ہûسأ´ضح¾«ء¶ؤ؟µؤ،£
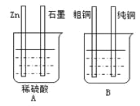
¢ظAةص±تا__(جî،°µç½â³ط،±»ٍ،°شµç³ط،±)،£
¢عئنضذZn½سBةص±ضذµؤ__£¬£¨جî،°´ضح،±»ٍ،°´؟ح،±£©£¬Bةص±ضذس¦¸أت¢__بـز؛،£
¢غ·ض±ًذ´³ِت¯ؤ«°ô؛ح´؟ح°ôµؤµç¼«·´س¦ت½
ت¯ؤ«°ô£؛___£¬
´؟ح°ô£؛__،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟Hagemannُ¥(H)تاز»ضض؛د³ة¶à»·»¯؛دخïµؤضذ¼نجه£¬؟ةسةدآءذآ·دك؛د³ة(²؟·ض·´س¦جُ¼آشب¥)£؛
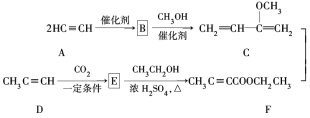
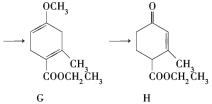
(1)A،ْBخھ¼س³ة·´س¦£¬شٍBµؤ½ل¹¹¼ٍت½تا________£»B،ْCµؤ·´س¦ہàذحتا_______،£
(2)Hضذ؛¬سذµؤ¹ظؤـحإأû³ئتا__________£»Fµؤأû³ئ(دµح³أüأû)تا_______،£
(3)E،ْFµؤ»¯ر§·½³جت½تا______________،£
(4)TMOBتاHµؤح¬·ضزى¹¹جه£¬¾كسذدآءذ½ل¹¹جطص÷£؛¢ظ؛ث´إ¹²صٌاâئ׳±½»·خüتص·هحâ½ِسذ1¸ِخüتص·ه£»¢ع´وشع¼×رُ»ù(CH3O£)،£TMOBµؤ½ل¹¹¼ٍت½تا________،£
(5)دآءذثµ·¨صب·µؤتا_______،£
a£®Aؤـ؛حHCl·´س¦µأµ½¾غآبززد©µؤµ¥جه
b£®D؛حFسأنهث®؟ةزش¼ّ±ً
c£®1 mol Gحêب«ب¼ةصةْ³ة7 mol H2O
d£®Hؤـ·¢ةْ¼س³ة،¢ب،´ْ·´س¦
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟25،وت±£¬سأ0.10mol/Lµؤرخثل·ض±ًµخ¶¨جه»دàح¬ازإ¨¶ب¾ùخھ0.10mol/Lµؤبضضز»شھ¼îXOH،¢YOH¼°ZOH£¬µخ¶¨اْدكبçح¼ثùت¾،£

دآءذثµ·¨صب·µؤتا
A. µخ¶¨XOHت±؟ةسأ·سجھ×÷ض¸ت¾¼ء
B. YOHتاا؟¼î
C. X++H2O![]() XOH+H+µؤئ½؛â³£تK=10-4
XOH+H+µؤئ½؛â³£تK=10-4
D. [V(رخثل)/V(¼î)]=0.5ت±£¬c(Z+)>c(Y+)>c(X+)
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟¾ف،¶؟ئ¼¼بص±¨،·±¨µہ£¬خز¹ْ؟ئر§¼زرذضئ³ة¹¦ز»دµءذت¯ؤ«د©دقسٍµؤ3d¹¶ة½ًتôضذذؤ(Mn،¢Fe،¢Co،¢Ni،¢Cu)´ك»¯¼ء£¬شعتزخآجُ¼دآزشH2O2خھرُ»¯¼ءض±½س½«CH4رُ»¯³ةCµؤ؛¬رُ»¯؛دخï،£اë»ط´ًدآءذختجâ£؛
£¨1£©شعMn،¢Fe،¢Co،¢Ni،¢Cuضذ£¬ؤ³»ùج¬ش×س؛ثحâµç×سإإ²¼×ٌر،°؛éجط¹وشٍجطہ،±£¨ض¸ؤـء؟دàح¬µؤش×س¹ىµہشعب«آْ،¢°ëآْ،¢ب«؟ص×´ج¬ت±£¬جهدµµؤؤـء؟×îµح£©£¬¸أش×سµؤحâخ§µç×سإإ²¼ت½خھ_____،£
£¨2£©شع3d¹¶ة½ًتôضذ£¬»ùج¬ش×سخ´³ة¶شµç×ست×î¶àµؤشھثطتا_____£¨جîشھثط·û؛إ£©،£
£¨3£©حµؤروة«·´س¦³تآجة«£¬شعدض´ْ»¯ر§ضذ£¬³£ہûسأش×س¹âئ×ةدµؤجطص÷ئ×دكہ´¼ّ¶¨شھثط£¬³ئخھ_____،£
£¨4£©ت¯ؤ«د©دقسٍµ¥ش×سجْؤـ»î»¯CH4·ض×سضذµؤC-H¼ü£¬µ¼ضآCسëHض®¼نµؤ×÷سأء¦_____ (،°¼ُبُ،±»ٍ،°²»±ن،±)،£جْ¾§جهضذء£×سض®¼ن×÷سأء¦ہàذحتا_____،£
£¨5£©³£خآدآ£¬H2O2رُ»¯CH4ةْ³ةCH3OH،¢HCHO،¢HCOOHµب،£
¢ظثüأاµؤ·ذµم·ض±ًخھ64.7،و،¢-19.5،و،¢100.8،و£¬ئنض÷زھشزٍتا_____£»
¢عCH4؛حHCHO±ب½د£¬¼ü½ا½د´َµؤتا_____£¬ض÷زھشزٍتا_____،£
£¨6£©îـ¾§°û؛ح°×ح(حؤّ؛د½ً)¾§°û·ض±ًبçح¼1،¢2ثùت¾،£
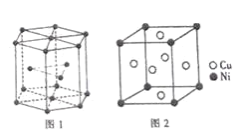
¢ظîـ¾§°û¶ر»·½ت½µؤأû³ئخھ_____£»
¢عزرضھ°×ح¾§°ûµؤأـ¶بخھdg،¤cm-3£¬NA´ْ±ي°¢·ü¼سµآآق³£تµؤضµ،£ح¼2¾§°ûضذء½¸ِأوذؤةدحش×س×î¶ج؛ث¼ن¾àخھ_____ pm(ءذ³ِ¼ئثمت½)،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟25 ،وت±£¬دآءذ¸÷×éہë×سشعض¸¶¨بـز؛ضذز»¶¨ؤـ´َء؟¹²´وµؤتا£¨،،،،£©
A.0.1 mol،¤L£1 AlCl3بـز؛ضذ£؛H£«،¢Na£«،¢Cl£،¢![]()
B.ؤـت¹×دة«ت¯بïبـز؛±نہ¶µؤبـز؛£؛Ag£«،¢Fe3£«،¢Br£،¢![]()
C.ؤـت¹µي·غµâ»¯¼طتشض½دشہ¶ة«µؤبـز؛£؛K£«،¢![]() ،¢S2£،¢
،¢S2£،¢![]()
D.سةث®µçہë²ْةْµؤc£¨H£«£©£½10£12 mol،¤L£1µؤبـز؛ضذ£؛![]() ،¢
،¢![]() ،¢
،¢![]() ،¢Cl£
،¢Cl£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟ذآذح؟ة؟طµç³ط،ھ،ھï®ث®µç³ط£¬¹¤×÷شہيبçح¼ثùت¾،£دآءذسذ¹طثµ·¨²»صب·µؤتا£¨،،،،£©
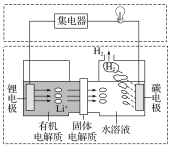
A.ج¼¼«·¢ةْµؤ·´س¦£؛2H2O£«2e£=H2،ü£«2OH£
B.سذ»ْµç½âضت؛حث®بـز؛²»؟ةزش»¥»»اّسٍ
C.ہيآغةدحâµçآ·ضذأ؟×ھزئ1 molµç×س£¬¸؛¼«دû؛ؤµؤضتء؟خھ7 g
D.بô¸أµç³ط؟ةزش³نµç£¬³نµçت±ج¼¼«½سحâ¼سµçش´µؤ¸؛¼«£¬ï®¼«½سحâ¼سµçش´µؤص¼«
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟ضـئع±يضذا°ثؤضـئعµؤشھثطA،¢B،¢C،¢D£¬ش×سذٍتزہ´خشِ´َ£¬ازA،¢B،¢Cح¬ضـئع،£A¹²سذء½¸ِش×س¹ىµہةدسذµç×س£¬ازµç×ستؤ؟دàح¬،£B،¢Cدàءع£¬ازCضذµؤخ´³ة¶شµç×ستخھ3¸ِ£¬Dتابثہà×îشçت¹سأµؤشھثط£¬²¢زشصâضضشھثطأüأûءثز»¸ِت±´ْ،£اë»ط´ًدآأوµؤختجâ£؛
£¨1£©A،¢B،¢Cµعز»µçہëؤـ´سذ،µ½´َµؤث³ذٍخھ£؛__________________£¨جîشھثط·û؛إ£©£¬Dµؤ¼غ²مµç×سإإ²¼ح¼خھ£؛_______________________،£
£¨2£©شع²»ح¬µؤخآ¶بدآ£¬AزشACl2؛ح¶¾غجه A2Cl4ء½ضضذخت½´وشع£¬¶¾غجهµؤ½ل¹¹ت½بçدآح¼ثùت¾£؛
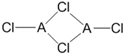
¢ظACl2ضذAµؤشس»¯·½ت½خھ_____________،£
¢ع1mol A2Cl4ضذ؛¬إنخ»¼üµؤتؤ؟خھ_____________،£
£¨3£©Bشھثطؤـذخ³ة¶àضضح¬ثطزىذخجه£¬ئنضذز»ضضح¬ثطزىذخجهXµؤ¾§جه½ل¹¹؛ح¾§°û½ل¹¹بçح¼ثùت¾،£زرضھXµؤأـ¶بتاa g/cm3£¬B-B¼üµؤ¼ü³¤خھr cm£¬°¢·ü¼سµآآق³£تµؤضµخھNA،£
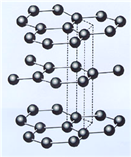
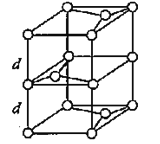
Xµؤ¾§جه½ل¹¹£¨×َ£©؛ح¾§°û£¨سز£©
¢ظXضذ؛¬سذµؤ»¯ر§¼üخھ____________________،£
¢عX¾§جهµؤ²م¼ن¾àہëخھ___________________،£
£¨4£©Cشھثط؟ةزشذخ³ةء½ضض؛¬رُثلHCO2؛حHCO3£¬ثلذشتاHCO3___HCO2£¨جî،°ا؟سع،±»ٍصك،°بُسع،±£©£¬شزٍخھ__________________________،£
£¨5£©½«¶خ¬أـضأ²مشعبخ¬؟ص¼نؤع¶ر»£¬؟ةزشµأµ½ء½ضض½ًتô¾§جهµؤ×îأـ¶ر»·½ت½،£ز»ضضتا°´صصXYXYXYXY،،·½ت½¶ر»£¬خزأا³ئصâضض¶ر»·½ت½خھ،°¼×،±·½ت½،£ءيحâز»ضضتا°´صصXYZXYZXYZXYZ،،·½ت½¶ر»£¬خزأا³ئصâضض¶ر»·½ت½خھ،°زز،±·½ت½،£شٍ½ًتôDµؤ¶ر»·½ت½خھ_______،££¨جî،°¼×،±»ٍ،°زز،±£©
²é؟´´ً°¸؛ح½âخِ>>
¹ْ¼تر§ذ£سإر، - ء·د°²لءذ±ي - تشجâءذ±ي
؛±±ت،»¥ءھحّخ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨ئ½ج¨ | حّةدسذ؛¦ذإد¢¾ظ±¨×¨اّ | µçذإص©ئ¾ظ±¨×¨اّ | ةوہْت·ذéخقض÷زهسذ؛¦ذإد¢¾ظ±¨×¨اّ | ةوئَاضب¨¾ظ±¨×¨اّ
خ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨µç»°£؛027-86699610 ¾ظ±¨ستدن£؛58377363@163.com