
����Ŀ���к�����ָ�������кͷ�Ӧ����lmol H2O���ų���������ijѧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ�����50mL0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ����ش��������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��_________���ձ���������������ĭ��������______��
��2�����ձ����粻��Ӳֽ�壬����õ��к�����ֵ______________������ƫ��������ƫС��������Ӱ������
��3��ʵ���и���60mL 0.50mol/L�������50mL 0.55mol/L��NaOH��Һ���з�Ӧ������ʵ����ȣ����ų�������_________�������������������������������к��ȵ���ֵ______��������������������������
���𰸡����β�������� ���¡����ȣ�����ʵ������е�������ʧ ƫС ����� ���
��������
��1���������ȼƵĹ������жϸ�װ�õ�ȱ���������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��2������Ӳֽ�壬����һ��������ɢʧ��
��3����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������DZ��¡����ȣ�����ʵ������е�������ʧ��
�ʴ�Ϊ�����β�������������¡����ȣ�����ʵ������е�������ʧ��
��2�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
��3����Ӧ�ų����������������Լ�������йأ�����60mL 0.50mol/L�������50mL 0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������ӣ����ų�������ƫ�ߣ������к�����ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������60mL 0.50mol/L�������50mL 0.55mol/L��NaOH��Һ��������ʵ�飬����к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��к����������������õĿ��������ʣ��Ը���ʵ��ش�
��1��ȷ��ȡ4.0g�ռ���Ʒ��
��2������Ʒ���250mL����Һ��
��3����____________�����������ƣ���ȡ25.00mL����Һ����ƿ�У����μӼ��μ�����ָʾ����
��4����0.2010 mol��L����������ζ������ռ���Һ���ζ�ʱ������ע��____________��ֱ���ζ��յ㡣�ﵽ�յ�ľ��������ǣ�____________��
��5��������ʵ��ζ����������±���
�ζ����� | ����Һ�����mL�� | �����������mL�� | |
�ζ�ǰ������mL�� | �ζ��������mL�� | ||
��һ�� | 25.00 | 0.50 | 20.40 |
�ڶ��� | 25.00 | 5.00 | 28.30 |
������ | 25.00 | 4.00 | 24.10 |
�������������ݣ������ռ�Ĵ��ȣ�____________
��6�����в����У��ᵼ������õ��ռ�Ĵ���ƫ�����________��
a���ζ��յ�ʱ�����ӿ̶�
b��û�����������Һ��ϴ��Ӧ�ĵζ���
c����ƿ��������������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�
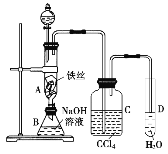
�ش��������⣺
(1)д��A�з�����Ҫ��Ӧ�Ļ�ѧ����ʽ___________________
(2)C��ʢ��CCl4��������_____________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����__________
(4)��Ҫ֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ�����ݲ�������ɫ����������֤�����㻹�����һ��������֤�ķ�����ָ��������Լ��ͳ��ֵ����ɡ�
������Լ�Ϊ________________
��Ӧ������Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��һ����ʵ��Ϊ������ѧ�ƣ�ʵ��̽���ܼ���ѧ��ѧϰ��ѧ����Ȥ��ij��ѧ��ȤС�������ͼʵ��װ�ã��г��豸���ԣ��Ʊ�������̽����������±��Ԫ�ص����ʡ��ش��������⣺

(1)����a��������______________��
(2)Aװ���з����Ļ�ѧ��Ӧ����ʽΪ_________________________________������Ư�ۻ���KClO3����Ӧ��ÿ����21.3g Cl2ʱת�Ƶĵ�����ĿΪ____NA��
(3)װ��B�����ڼ��ʵ�������C���Ƿ��������C�������˶�������B�пɹ۲쵽__________��
(4)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���ʱC�Т������������οɷ���____����ѡ��a��b��c����
ѡ�� | �� | �� | �� |
a | �������ɫ���� | Ũ���� | ʪ�����ɫ���� |
b | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
c | ʪ�����ɫ���� | ��ʯ�� | �������ɫ���� |
(5)���װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ���ɹ۲쵽��ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����壬��������D��������Һ����E�У���E���۲쵽��������_______________________________��������_____����������������������˵����ķǽ�����ǿ�ڵ⣬ԭ����_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2.5mol A��2.5mol B���ʢ���ݻ�Ϊ2L���ܱ�������������·�Ӧ��3A��g����B��g��![]() xC��g����2D��g������5s��Ӧ��ƽ�⣬�ڴ�5s��C��ƽ����Ӧ����Ϊ0.2mol��L��1��s��1��ͬʱ����1mol D�����������д������
xC��g����2D��g������5s��Ӧ��ƽ�⣬�ڴ�5s��C��ƽ����Ӧ����Ϊ0.2mol��L��1��s��1��ͬʱ����1mol D�����������д������
A. x��4
B. �ﵽƽ��״̬ʱ�����������ѹǿ����ʼʱѹǿ��Ϊ6��5
C. 5s��B�ķ�Ӧ����V��B����0.05mol��L��1��s��1
D. �ﵽƽ��״̬ʱA��ת����Ϊ50%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����á���ҵ�Ͽ�����ú���������ˮú�����ϳɶ�����( CH3OCH3)����ش��������⣺
(1)����ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2(g)+CO(g)![]() CH3OH(g) ��H= ��90��8kJ/mol
CH3OH(g) ��H= ��90��8kJ/mol
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
��CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H=��41��3kJ/mol
CO2(g)+H2(g) ��H=��41��3kJ/mol
�ܷ�Ӧ��3H2(g)+3CO(g)![]() CH3OCH3(g)+CO2(g) ����H= ��
CH3OCH3(g)+CO2(g) ����H= ��
һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�ǣ�________������ĸ���ţ���
a��ѹ����� b��������� c������CO2��Ũ�� d������CO��Ũ��e������������ѣ�CH3OCH3��
(2)��֪��Ӧ��2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ�ȣ�mol��L-1�� | 0��40 | 0��6 | 0��6 |
�ٱȽϴ�ʱ�����淴Ӧ���ʵĴ�С�Ƚϣ�![]() _________
_________![]() ������>������<������=������
������>������<������=������
�ڸ÷�Ӧ��ƽ�ⳣ���ı���ʽΪK=_____,�¶����ߣ��÷�Ӧ��ƽ�ⳣ��K____������������������С��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������֤����ӦN2(g)��3H2(g)![]() 2NH3(g)�Ѵﵽƽ��״̬����( ��)
2NH3(g)�Ѵﵽƽ��״̬����( ��)
A.1��N��N�����ѵ�ͬʱ����3��H��H������
B.1��N��N�����ѵ�ͬʱ����3��H��H���γ�
C.3��H��H�����ѵ�ͬʱ����6��N��H���γ�
D.1��N��N�����ѵ�ͬʱ����6��N��H���γ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1.0 mol��L��1�ļ���Һ����μ���1.0 mol��L��1������Һ����������������������������������������Һ�������ϵ��ͼ��ʾ��
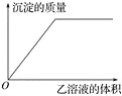
����ͼ�й�ϵ�������и�����Һ�е�(����)
A. ��Ϊ�Ȼ�������Ϊ�������� B. ��Ϊ�������ƣ���Ϊ�Ȼ���
C. ��Ϊƫ�����ƣ���Ϊ���� D. ��Ϊ�Ȼ�������Ϊ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����գ�
(1)����ͬ�¶Ⱥ�ѹǿ�µ���������12C18O��14N2�������������������ͬ������������������������֮��Ϊ__________�������������ԭ������ȣ���������������������֮��_________������������������ͬ��������������ܶ�֮��Ϊ_________��
(2)10.8 g R2O5����ԭ�ӵ���ĿΪ3.01��1023����Ԫ��R�����ԭ������Ϊ ________��
(3)��10 mL1.00 mol/L Na2CO3��Һ��10 mL1.00 mol/L CaCl2��Һ���ͣ�������Һ��Na+�����ʵ���Ũ��Ϊ___________�����Ի��ǰ����Һ����ı仯����
(4)�ڱ�״���£���CO��CO2��ɵĻ������Ϊ6.72 L������Ϊ12 g���˻������CO��CO2���ʵ���֮����_________��
(5)�ڿ���������CoC2O4�����ܵ��������CO2����ó�����պ��������Ϊ2.41g��CO2�����Ϊ1.344L(��״��)������������Ļ�ѧʽΪ_______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com