
����Ŀ������ʪ���Ʊ������һ�ֹ����������£�

��֪��������Ҫ�ɷ�ΪCa5(PO4)3(OH)��������Ca5(PO4)3F���л�̼�ȡ�
�ܽ�ȣ�Ca5(PO4)3(OH)<CaSO4��0.5H2O
��1�������������ܼӿ췴Ӧ���ʵĴ�ʩ��__________��
��2����������ʱ������Ӧ��
2Ca5(PO4)3(OH)+3H2O+10H2SO4![]() 10CaSO4��0.5H2O+6H3PO4
10CaSO4��0.5H2O+6H3PO4
�ٸ÷�Ӧ���ֳ����Թ�ϵ��H3PO4__________H2SO4���>����<������
�ڽ��Ԫ�������ɽ��͢��н��ۣ�P��S���Ӳ�����ͬ��__________��
��3�����ʱ��������Ca5(PO4)3F������ת��ΪHF������һ��ת��ΪSiF4��ȥ��д������HF�Ļ�ѧ����ʽ��__________��
��4��H2O2���������е��л�̼����ΪCO2�ѳ���ͬʱ����Ҳ�ᷢ���ֽ⡣��ͬͶ�ϱȡ���ͬ��Ӧʱ�䣬��ͬ�¶��µ��л�̼�ѳ�����ͼ��ʾ��80����ѳ��ʱ仯��ԭ��____________________��

��5������ʱ��CaCO3�Թ�������ַ�Ӧ������SO42������ԭ����__________������BaCO3�ɽ�һ���������ѳ��ʣ������ӷ���ʽ��____________________��
��6��ȡa g���þ������ᣬ������ˮϡ�ͣ��������̪��ָʾ������b mol��L1NaOH��Һ�ζ����յ�ʱ����Na2HPO4������NaOH��Һc mL������������H3PO4������������________������֪��H3PO4Ħ������Ϊ98 g��mol1��
���𰸡� ��ĥ������ �� �˵����P��S��ԭ�Ӱ뾶P��S���õ�������P��S���ǽ�����P��S 2Ca5(PO4)3F+10H2SO4+5H2O![]() 10CaSO4��0.5H2O+6H3PO4+2HF�� 80 ���H2O2�ֽ����ʴ�Ũ���������� CaSO4�� BaCO3+
10CaSO4��0.5H2O+6H3PO4+2HF�� 80 ���H2O2�ֽ����ʴ�Ũ���������� CaSO4�� BaCO3+![]() +2H3PO4
+2H3PO4![]() BaSO4+CO2��+H2O+2
BaSO4+CO2��+H2O+2![]()
![]()
���������������������������ɴ��������ʯ�࣬�����ᾭ�����л�̼������Ȳ����þ������ᡣ
��1��������������Ի�ѧ��Ӧ���ʵ�Ӱ��������������ܼӿ췴Ӧ���ʵĴ�ʩ�У���ĥ�����ȡ�
��2����������ǿ�����������ĸ��ֽⷴӦ���ɣ����ԣ�H3PO4![]() H2SO4��
H2SO4��
����Ԫ�������ɽ��ͣ�P��S���Ӳ�����ͬ���˵����P![]() S��ԭ�Ӱ뾶P
S��ԭ�Ӱ뾶P![]() S���õ�������P
S���õ�������P![]() S���ǽ�����P
S���ǽ�����P![]() S��
S��
��3��������ǿ�����������ĸ��ֽⷴӦ���ɣ�Ca5��PO4��3F��H2SO4��Ӧ����HF����ʯ������ᡣ
��4��ͼʾ����ͬͶ�ϱȡ���ͬ��Ӧʱ�䣬��ͬ�¶��µ��л�̼�ѳ��ʣ�80��ǰ�¶����߷�Ӧ���ʼӿ죬��ͬʱ�����л�̼�ѳ���������80����¶����ߣ�H2O2�ֽ����ʴ�H2O2Ũ���������ͣ���Ӧ���ʼ�������ͬ�������л�̼�ѳ��ʼ�С��
��5������ʱ��CaCO3�Թ�������ַ�Ӧ������SO42-������ԭ���ǣ�CaSO4����ˮ������BaCO3�ɽ�һ���������ѳ��ʣ���ΪBaSO4������ˮ����Ӧ�����ӷ���ʽΪBaCO3+SO42-+2H3PO4=BaSO4+CO2��+2H2PO4-+H2O��
��6�����������ϵʽΪH3PO4~2NaOH�������ĵ�NaOH����H3PO4��
�������1����ĥ������Ӧ��ĽӴ�������ӿ췴Ӧ���ʣ������������¶ȼӿ췴Ӧ���ʣ��������ܼӿ췴Ӧ���ʵĴ�ʩ�У���ĥ�����ȡ�
��2����������ǿ�����������ĸ��ֽⷴӦ���ɣ����ԣ�H3PO4![]() H2SO4��
H2SO4��
����Ԫ�������ɽ������ԣ�H3PO4![]() H2SO4��P��S���Ӳ�����ͬ���˵����P
H2SO4��P��S���Ӳ�����ͬ���˵����P![]() S��ԭ�Ӱ뾶P
S��ԭ�Ӱ뾶P![]() S���õ�������P
S���õ�������P![]() S���ǽ�����P
S���ǽ�����P![]() S��
S��
��3��������ǿ�����������ĸ��ֽⷴӦ���ɣ�Ca5��PO4��3F��H2SO4��Ӧ����HF����ʯ������ᣬ����HF�Ļ�ѧ����ʽΪ2Ca5��PO4��3F+10H2SO4+5H2O![]() 10CaSO4��0.5H2O+6H3PO4+2HF����
10CaSO4��0.5H2O+6H3PO4+2HF����
��4��ͼʾ����ͬͶ�ϱȡ���ͬ��Ӧʱ�䣬��ͬ�¶��µ��л�̼�ѳ��ʣ�80��ǰ�¶����߷�Ӧ���ʼӿ죬��ͬʱ�����л�̼�ѳ���������80����¶����ߣ�H2O2�ֽ����ʴ�H2O2Ũ���������ͣ���Ӧ���ʼ�������ͬ�������л�̼�ѳ��ʼ�С��
��5������ʱ��CaCO3�Թ�������ַ�Ӧ������SO42-������ԭ���ǣ�CaSO4����ˮ������BaCO3�ɽ�һ���������ѳ��ʣ���ΪBaSO4������ˮ������SO42-��BaCO3���ɸ����ܵ�BaSO4��CO32-��H3PO4������ǿ��H2CO3���ڴ�������CO32-ת����H2O��CO2����Ӧ�����ӷ���ʽΪBaCO3+SO42-+2H3PO4=BaSO4+CO2��+2H2PO4-+H2O��
��6���ζ��յ�����Na2HPO4�������ĵ�H3PO4��NaOH���ʵ���֮��Ϊ1��2��n��H3PO4��=![]() n��NaOH��=
n��NaOH��=![]() bmol/L
bmol/L![]() c
c![]() 10-3L=
10-3L=![]() mol��m��H3PO4��=
mol��m��H3PO4��=![]() mol
mol![]() 98g/mol=
98g/mol=![]() g=0.049bcg������������H3PO4����������Ϊ
g=0.049bcg������������H3PO4����������Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵����ȷ����
A. 298Kʱ����Ӧ2Mg(s)��CO2(g)===C(s)��2MgO(s)���Է����У���÷�Ӧ�Ħ�H>0
B. ��ⷨ����ͭʱ����ͭ����������ͭ������
C. �����ᱵ����Һ�м�����������Na2CO3��Һ�����������ó����м������������������˵��Ksp(BaSO4)>Ksp(BaCO3)
D. �����£�pH��Ϊ5���������Ȼ����Һ�У�ˮ�ĵ���̶���ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)ͬ��ͬѹ�£�ͬ�����N2��SO2������֮��Ϊ__________________�����ʵ���֮��Ϊ____________��ԭ������֮��Ϊ____________��Ħ������֮��Ϊ__________������֮��Ϊ________________���ܶ�֮��Ϊ______________��
(2)����������Ϊ11��14��CO2��CO�Ļ�����壬��û�������е�CO2��CO���ʵ���֮��Ϊ________��̼ԭ�Ӻ���ԭ�ӵĸ�����Ϊ________���û�������Ħ������Ϊ________��
(3)12.4gNa2X�к�Na+ 0.4mol����Na2X��Ħ��������________��X�����ԭ��������________��
(4)�������(�����ͺ����Ļ����)�����ڼ���ijЩ��������ˮDZˮԱʹ�á��ڱ�״���£�11.2L�������������������4.8g�����������ͺ����ķ�����֮����______��������������_______��
(5)ijԪ�ص�һ��ԭ�ӵ�����Ϊag��һ��12Cԭ�ӵ�����Ϊbg�������ӵ�����ΪNA�����ԭ�ӵ����ԭ��������ֵ�ɱ�ʾΪ______________��_______________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������Fe��FeO��![]() �Ļ�����м���100 mLŨ��Ϊ
�Ļ�����м���100 mLŨ��Ϊ![]() �����ᣬ����ǡ����ȫ��Ӧ���ų�224 mL������
�����ᣬ����ǡ����ȫ��Ӧ���ų�224 mL������![]() ��״��
��״��![]() ��������Һ�м���KSCN��Һ����Ѫ��ɫ���֡�����������CO�ڸ����»�ԭ��ͬ�����Ĵ˻����ܵõ�����������
��������Һ�м���KSCN��Һ����Ѫ��ɫ���֡�����������CO�ڸ����»�ԭ��ͬ�����Ĵ˻����ܵõ�����������![]() ��
��![]()
A. ![]() B.
B. ![]() C.
C. ![]() D.
D. ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������![]() �ķ��ӽṹ������˵����ȷ���ǣ� ��
�ķ��ӽṹ������˵����ȷ���ǣ� ��
A. �������������̼ԭ���п��ܶ���һ��ֱ����
B. �������������̼ԭ�Ӳ����ܶ���һ��ֱ����
C. 12��̼ԭ�Ӳ����ܶ���ͬһƽ����
D. ����ԭ���п��ܶ���ͬһƽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼʾ��ʾ����X��Y��Z��W��һ�������¿����ռ�ͷһ��ת����
��� | X | Y | Z | W |
|
��1�� | |||||
��2�� |
|
| |||
��3�� |
|
|
��1��X�ǽ������ʣ���ɫ��Ӧ�ʻ�ɫ��Y�Ĵ������׳��⣻Z����������θ����ࣻX�ڻ���ɫ��������ȼ�տ��Բ�������W����֪���������̬��W���Եõ�X����
X�ǣ�___________Y�ǣ�___________Z�ǣ�__________W�ǣ�__________����д��ѧʽ��
��2��Z����Ư���ԣ����Dz��ȶ����ֽ�������Ȼ����Z�ǣ�________����д��ѧʽ��
��3������X��Y���Եμ�___________��Һ��ǰ�߳���Ѫ��ɫ������д���ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩ���첫���ϳ�·���У����е�һ��ת�����£�
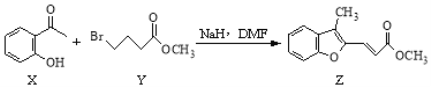
����˵����ȷ����
A. Y�ķ���ʽΪC5H9O2Br
B. X����������ԭ����ͬһƽ����
C. Z������H2�ӳɺ�ķ�������5������̼ԭ��
D. �����ʵ�����X��Z�ֱ�����ˮ��Ӧ���������Br2�����ʵ���֮��1:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��������Ʒ�к�������������������ijͬѧҪ�ⶨ������Ԫ�ص��������������������ʵ�鷽����

(1)��������������Һʱ�����õ��IJ����������ձ�����Ͳ������������ͷ�ι����⣬��������__________(����������)��
(2)��Ӧ���м�������H2O2��Һ����Ӧ�����ӷ���ʽ��_______________________________��
(3)���������![]() �Ƿ�ϴ�Ӹɾ��IJ�����_______________________________________��
�Ƿ�ϴ�Ӹɾ��IJ�����_______________________________________��
(4)���������ȣ���ȴ�����£�����ƽ������������Ⱥ�����������Ϊb1g���ٴμ��Ȳ���ȴ�����³�������Ϊb2g����b1��b2��0.3����Ӧ���еIJ�����_____________________��
(5)����������Ϊ42.6 g��������������Ⱥ�Ĺ����������Ϊ45.8 g������Ʒ����Ԫ�ص���������Ϊ________________��
(6)��ͬѧ��Ϊ����������ʵ�鲽��̫����������Ϊ��ֻҪ����Ʒ����ˮ��ֽ��裬���ȡ����ɡ����ճ������ɲ����Ʒ����Ԫ�ص���������������Ϊ������������Ƿ���У�__________(����С������С�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������������װ����FeCl3���ɹ�ѡ����Լ��У���MnO2����NaOH��Һ���۱���NaCl��Һ����Ũ���ᡡ��Ũ���ᡣ

(1)�������������������У���װ�õ�����˳��Ϊ(��дA��E���)��__��__��___��__��__��
(2)װ�����Ӻú�Ӧ�����Ƚ��е�ʵ�������____________________________________________��
(3)Aװ����ƿ�з�Ӧ�����ӷ���ʽ��_________________________________________��
(4)E��ʢװ���Լ���________����������_________________________________________��
(5)ֹͣ��Ӧ������˿ʣ�ࡣΪ����FeCl3�����ɣ������յõ�FeCl3��Һ����ͬѧ�������ʵ�鲽�裺
a����Bװ�ò�������ȴ������������ˮ�ܽ⣬________(���������)��ȥ�����
b��ȡ������Һ���μ�________��Һ����Һ���ֺ�ɫ���Դ˼���Fe3����
c��ȡ������Һ���μ������ữ��AgNO3��Һ������Cl����
(6)��ͬѧ��Ϊ��ʵ����Ʋ������յõ�FeCl3��Һ����������(д����Ӧ�����ӷ�Ӧ����ʽ)__________________________________________________________________��
����Ϊ��ѡ��������Щ�Լ������Ƶý�Ϊ������FeCl3��Һ________��
A��KMnO4(H��) B��Fe����C��H2O2 D��Cl2 ����E������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com