
ЁОЬтФПЁПжиИѕЫсФЦЫзГЦКьЗЏФЦ![]() ЃЌЪЧживЊЕФЛЏЙЄВњЦЗКЭЧПбѕЛЏМСЃЎЙЄвЕжЦБИКьЗЏФЦЕФСїГЬШчЯТЃК
ЃЌЪЧживЊЕФЛЏЙЄВњЦЗКЭЧПбѕЛЏМСЃЎЙЄвЕжЦБИКьЗЏФЦЕФСїГЬШчЯТЃК

(1)ЛЏбЇЩЯПЩНЋФГаЉбЮаДГЩбѕЛЏЮяЕФаЮЪНЃЌШч![]() ПЩаДГЩ
ПЩаДГЩ![]() ЃЌдђ
ЃЌдђ![]() ПЩаДГЩ______ЃЎ
ПЩаДГЩ______ЃЎ
(2)ьбЩеИѕЬњПѓЪБЃЌПѓЪЏжаФбШмЕФ![]() ЩњГЩПЩШмгкЫЎЕФ
ЩњГЩПЩШмгкЫЎЕФ![]() ЃЌЗДгІЛЏбЇЗНГЬЪНШчЯТЃК
ЃЌЗДгІЛЏбЇЗНГЬЪНШчЯТЃК![]() ЮЊСЫМгПьИУЗДгІЕФЗДгІЫйТЪЃЌПЩВЩШЁЕФДыЪЉЪЧ______
ЮЊСЫМгПьИУЗДгІЕФЗДгІЫйТЪЃЌПЩВЩШЁЕФДыЪЉЪЧ______![]() аДвЛжжМДПЩ
аДвЛжжМДПЩ![]()
(3)вбжЊ![]() дкВЛЭЌЕФЫсадШмвКжагаВЛЭЌЕФЗДгІЃЌШчЃК
дкВЛЭЌЕФЫсадШмвКжагаВЛЭЌЕФЗДгІЃЌШчЃК
![]() ЃЛ
ЃЛ![]()
ЂйЭљЛьКЯШмвКМзжаМгШыСђЫсБиаыЪЪСПЕФдвђЪЧ______ЃЎ
ЂкЛьКЯШмвКввжаШмжЪЕФЛЏбЇЪНЪЧ______ЃЎ
(4)дкКЌ![]() ЗЯЫЎжаДцдкзХЦНКтЃК
ЗЯЫЎжаДцдкзХЦНКтЃК![]() ЃЌЧыаДГіИУЦНКтЕФЦНКтГЃЪ§БэДяЪН
ЃЌЧыаДГіИУЦНКтЕФЦНКтГЃЪ§БэДяЪН![]() ______ЃЌШєМЬајМгЫЎЯЁЪЭЃЌЦНКтНЋ______вЦЖЏ
______ЃЌШєМЬајМгЫЎЯЁЪЭЃЌЦНКтНЋ______вЦЖЏ![]() ЬюЁАе§ЯђЁБЁЂЁАФцЯђЁБЁАВЛЁБ
ЬюЁАе§ЯђЁБЁЂЁАФцЯђЁБЁАВЛЁБ![]() ЃЎ
ЃЎ
(5)ЧыХфЦНМюадШмвКЛЙдЗЈжаЗЂЩњЕФРызгЗДгІЃК
______![]() ___
___![]() ______
______![]() --______
--______![]() ______
______![]() ______
______![]() ЃЎ
ЃЎ
ЁОД№АИЁП![]() ЗлЫщПѓЪЏЁЂЩ§ИпЮТЖШ ЩйСПВЛФмГ§ОЁ
ЗлЫщПѓЪЏЁЂЩ§ИпЮТЖШ ЩйСПВЛФмГ§ОЁ![]() ЕШдгжЪЃЌЙ§СПЛсЩњГЩ
ЕШдгжЪЃЌЙ§СПЛсЩњГЩ![]() ЕШИБВњЮя
ЕШИБВњЮя ![]() КЭ
КЭ![]()
![]() е§Яђ 4 6 19 8 3 14
е§Яђ 4 6 19 8 3 14
ЁОНтЮіЁП
ИѕЬњПѓМгШыДПМюЃЌЭЈШыПеЦјьбЩеЃЌОНўШЁКѓЕУЕНNa2CrO4ЁЂNa2CO3ЃЌМгШыЪЪСПСђЫсЃЌЕУЕНЛьКЯШмвКввКЌгаNa2Cr2O7КЭNa2SO4ЃЌШЛКѓНсОЇПЩЕУЕНNa2Cr2O7ОЇЬхЃЌ
ЃЈ1ЃЉРрБШNa2SiO3ПЩаДГЩNa2OSiO2ЭъГЩFeЃЈCrO2ЃЉ2ЕФбѕЛЏЮяЕФаЮЪНЃЛ
ЃЈ2ЃЉИљОнгАЯьЛЏбЇЗДгІЫйТЪЕФвђЫиЗжЮіЃЛ
ЃЈ3ЃЉЂйЙЬЬхЭМЪОМАЬтжааХЯЂПЩжЊЃЌЩйСПВЛФмГ§ОЁNa2CO3ЕШдгжЪЃЌЙ§СПЛсЩњГЩNa2Cr3O10ЕШИБВњЮяЃЛ
ЂкЗЂЩњСЫЗДгІ2CrO42-+2H+=Cr2O72-+H2OЃЌЩњГЩСЫNa2Cr2O7КЭNa2SO4ЃЛ
ЃЈ4ЃЉЛЏбЇЦНКтГЃЪ§ЃЌЪЧжИдквЛЖЈЮТЖШЯТЃЌПЩФцЗДгІДяЕНЦНКтЪБИїЩњГЩЮяХЈЖШЕФЛЏбЇМЦСПЪ§ДЮУнЕФГЫЛ§Г§вдИїЗДгІЮяХЈЖШЕФЛЏбЇМЦСПЪ§ДЮУнЕФГЫЛ§ЫљЕУЕФБШжЕЃЌЫЎВЛашвЊаДГіЃЌМгЫЎДйНјЦНКте§ЯђвЦЖЏЃЛ
ЃЈ5ЃЉCr2O72-ЁњCr3+ЃЌS2-ЁњS2O32-ЃЌИљОнЕчзгзЊвЦЪиКуХфЦНЁЃ
ИѕЬњПѓМгШыДПМюЃЌЭЈШыПеЦјьбЩеЃЌОНўШЁКѓЕУЕНNa2CrO4ЁЂNa2CO3ЃЌМгШыЪЪСПСђЫсЃЌЕУЕНЛьКЯШмвКввКЌгаNa2Cr2O7КЭNa2SO4ЃЌШЛКѓНсОЇПЩЕУЕНNa2Cr2O7ОЇЬхЃЌ
(1)Fe(CrO2)2жаЬњдЊЫиЛЏКЯМлЪЧ+2МлЃЌбѕЛЏЮяЮЊFeOЃЌИѕдЊЫиЛЏКЯМл+3МлЃЌбѕЛЏЮяЮЊCr2O3ЃЌЫљвдFe(CrO2)2аДГЩбѕЛЏЮяаЮЪНЮЊFeOCr2O3ЃЌЙЪД№АИЮЊЃКFeOCr2O3ЃЛ
(2)діДѓЗДгІЮяНгДЅУцЛ§ПЩвдМгПьЗДгІЫйТЪЃЌЩ§ИпЮТЖШПЩвдДѓДѓМгПьЗДгІЫйТЪЃЌЙЪД№АИЮЊЃКЗлЫщПѓЪЏЁЂЩ§ИпЮТЖШЃЛ
(3)ЂйгЩгкЩйСПФбвдГ§ОЁЬМЫсФЦЃЌЙ§СПЛсЗЂЩњЗДгІ3CrO42+4H+=Cr3O102+2H2OЃЌЩњГЩСЫNa2Cr3O10ЕШИБВњЮяЃЌЫљвдБиаыМгШыЪЪСПСђЫсЃЌЙЪД№АИЮЊЃКЩйСПВЛФмГ§ОЁ![]() ЕШдгжЪЃЌЙ§СПЛсЩњГЩ
ЕШдгжЪЃЌЙ§СПЛсЩњГЩ![]() ЕШИБВњЮяЃЛ
ЕШИБВњЮяЃЛ
ЂкгЩгкЗЂЩњСЫЗДгІ2CrO42+2H+=Cr2O72+H2OЃЌЩњГЩСЫ![]() КЭ
КЭ![]() ЃЌЫљвдввжаШмжЪЕФЛЏбЇЪН
ЃЌЫљвдввжаШмжЪЕФЛЏбЇЪН![]() КЭ
КЭ![]() ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК![]() КЭ
КЭ![]() ЃЛ
ЃЛ
(4)ИљОнЛЏбЇЦНКтГЃЪ§ЕФЖЈвхЃЌПЩжЊCr2O72(aq)+H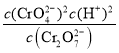 ЃЌМгЫЎЯЁЪЭЃЌДйНјЫЎНтЃЌЦНКте§ЯђвЦЖЏЃЌЙЪД№АИЮЊЃК
ЃЌМгЫЎЯЁЪЭЃЌДйНјЫЎНтЃЌЦНКте§ЯђвЦЖЏЃЌЙЪД№АИЮЊЃК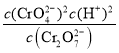 ЃЛе§ЯђЃЛ
ЃЛе§ЯђЃЛ
(5) Cr2O72ЁњCr3+ЃЌ1mol Cr2O72ЛёЕУЕчзгЮяжЪЕФСПЮЊ1molЁС2ЁС(63)=6molЃЌS2ЁњS2O32ЃЌ2molS2ЪЇШЅЕчзгЮяжЪЕФСПЮЊ1molЁС2ЁС[2(2)]=8molЃЌИљОнЕчзгзЊвЦЪиКуЃЌзюаЁЙЋБЖЪ§ЮЊ24ЃЌЫљвдЗЂЩњЕФРызгЗДгІЮЊЃК4Cr2O72+6S2+19H2OЈT8Cr(OH)3Ё§+3S2O32+14OHЃЌЙЪД№АИЮЊЃК4ЁЂ6ЁЂ19ЁЂ8ЁЂ3ЁЂ14ЁЃ


 УћЪІЕМКНЕЅдЊЦкФЉГхДЬ100ЗжЯЕСаД№АИ
УћЪІЕМКНЕЅдЊЦкФЉГхДЬ100ЗжЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГХЈЖШЕФАБЫЎжаДцдкЯТСаЦНКтЃКNH3ЁЄH2O![]()
![]() ЃЋOHЃЃЌШчЯыМѕаЁ
ЃЋOHЃЃЌШчЯыМѕаЁ![]() ЕФХЈЖШЃЌЖјдіДѓOHЃЕФХЈЖШЃЌВЩШЁДыЪЉгааЇЕФЪЧ(ЁЁЁЁ)
ЕФХЈЖШЃЌЖјдіДѓOHЃЕФХЈЖШЃЌВЩШЁДыЪЉгааЇЕФЪЧ(ЁЁЁЁ)
A.ЭЈШыHClB.МгШыNH4ClЙЬЬх
C.МгШыЩйСПFeCl3D.МгШыЩйСПNaOH
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГ![]() бљЦЗжаКЌга
бљЦЗжаКЌга![]() КЭ
КЭ![]() дгжЪЃЌЯжгћжЦШЁДПОЛЕФ
дгжЪЃЌЯжгћжЦШЁДПОЛЕФ![]() ЃЌФГЭЌбЇЩшМЦШчЭМЕФЪЕбщЗНАИЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЌФГЭЌбЇЩшМЦШчЭМЕФЪЕбщЗНАИЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЪЕбщЗНАИЃК

(1)ВйзїЂёЕФУћГЦЪЧ______ЃЌдкИУВйзїжагУЕНЕФВЃСЇвЧЦїГ§СЫгаЩеБЁЂВЃСЇАєЃЌЛЙга______ЁЃ
(2)ГСЕэAЕФГЩЗжЪЧ______![]() ЬюЛЏбЇЪН
ЬюЛЏбЇЪН![]() ЃЌаДГіЕкЂлВНЗДгІжаТСдЊЫизЊЛЏЕФРызгЗНГЬЪН______ЁЃ
ЃЌаДГіЕкЂлВНЗДгІжаТСдЊЫизЊЛЏЕФРызгЗНГЬЪН______ЁЃ
(3)аДГіжЄУїТЫвКBжа![]() вбГСЕэЭъШЋЕФЪЕбщЗНЗЈ______ЁЃ
вбГСЕэЭъШЋЕФЪЕбщЗНЗЈ______ЁЃ
(4)ВЛИФБфЩЯЪіСїГЬЭМЕФНсЙЙЃЌНЋЁАЂйЙ§СПбЮЫсЁБЁАЂкЙ§СПNaOHЁБНЛЛЛЮЛжУЃЌдђЁАЂлЙ§СП![]() ЁБгІИФЮЊ__________ЃЌаДГіДЫЗНАИЯТЩњГЩГСЕэBЕФРызгЗНГЬЪН______ЁЃ
ЁБгІИФЮЊ__________ЃЌаДГіДЫЗНАИЯТЩњГЩГСЕэBЕФРызгЗНГЬЪН______ЁЃ
(5)ЮЊСЫЕУЕНИќМгДПОЛЕФ![]() ЃЌЙ§ТЫКѓашвЊНјааВйзїВНжшЪЧ______ЁЃ
ЃЌЙ§ТЫКѓашвЊНјааВйзїВНжшЪЧ______ЁЃ
(6)аДГіЙЄвЕгЩбѕЛЏТСвБСЖТСЕФЛЏбЇЗНГЬЪН______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПГЃЮТЯТЃЌФГбЇЩњгУ0.1 molЁЄLЃ1H2SO4ШмвКЕЮЖЈ0.1 molЁЄLЃ1NaOHШмвКЃЌжаКЭКѓМгЫЎжС100 mLЁЃШєЕЮЖЈжеЕуЕФХаЖЈгаЮѓВюЃКЂйЩйЕЮСЫвЛЕЮH2SO4ШмвКЃЛЂкЖрЕЮСЫвЛЕЮH2SO4ШмвК(1ЕЮЮЊ0.05 mL)ЃЌдђЂйКЭЂкСНжжЧщПіЯТЫљЕУШмвКЕФpHжЎВюЪЧ(ЁЁЁЁ)
A. 4B. 4.6C. 5.4D. 6
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП2018Фъ4дТ23ШеЃЌжаЙњШЫУёКЃОќГЩСЂ69жмФъЁЃЬсЕНКЃОќОЭВЛЕУВЛЬсКНПеФИНЂЃЌЮвЙње§дкНЈдьЕкШ§ЫвКНПеФИНЂЁЃФјИѕИжПЙИЏЪДадФмЧПЃЌПЩгУгкНЈдьКНФИЁЃ
(1)КНФИМзАхФјИѕИжБэУцЭПгавЛВуФЭИпЮТЕФВФСЯОлЙшбѕЭщЃЈНсЙЙШчЭММзЫљЪОЃЉЁЃЛљЬЌCrдзгЕФМлЕчзгХХВМЪНЮЊ_____________ЃЌЛљЬЌSiдзгЕчзгеМОнзюИпФмМЖЕФЕчзгдЦТжРЊЭМЮЊ_____________аЮЃЌдЊЫиCЁЂOЁЂFЕФЕчИКадгЩДѓЕНаЁЕФЫГађЮЊ_____________ЁЃ
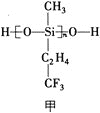
(2)КЃбѓЪЧдЊЫиЕФвЁРКЃЌКЃЫЎжаКЌгаДѓСПFЁЂClЁЂBrЁЂIдЊЫиЁЃ
ЂйOF2ЕФПеМфЙЙаЭЮЊ_____________ЃЌЦфжаOдзгдгЛЏЗНЪНЮЊ_____________дгЛЏЁЃ
ЂкKClОЇЬхжаДцдкЕФЛЏбЇМќРраЭга_____________ЃЛCaCl2ШлЕувЊБШKClЕФШлЕуИпКмЖрЃЌжївЊдвђЮЊ____________________________________________________________________ЁЃ
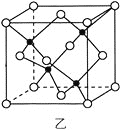
(3)КЃЕзН№ЪєШэФрЪЧдкКЃбѓЕзИВИЧзХЕФвЛВуКьзиЩЋГСЛ§ЮяЃЌдЬВизХДѓСПЕФзЪдДЃЌКЌгаЙшЁЂбѕЛЏЬњЁЂУЬЁЂаПЕШЁЃZn2+гыS2-аЮГЩЕФвЛжжОЇАћНсЙЙШчЭМввЫљЪОЃЈКкЧђБэЪОZn2+ЃЌАзЧђБэЪОS2-ЃЉЃЌZn2+ЕФХфЮЛЪ§ЮЊ_____________ЁЃОЇАћБпГЄЮЊa nmЁЂZnSЯрЖдЗжзгжЪСПЮЊMЃЌАЂЗќМгЕТТоГЃЪ§ЕФжЕЮЊNAЃЌЦфОЇЬхУмЖШЕФМЦЫуБэДяЪНЮЊ_____________gЁЄcm-3ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖўСђЛЏют(MoS2ЃЌ ЦфжаMoЕФЛЏКЯМлЮЊ+4)БЛгўЮЊЁАЙЬЬхШѓЛЌМСжЎЭѕЁБЃЌРћгУЕЭЦЗжЪЕФЛдютПѓ(КЌMoS2ЁЂSiO2 вдМАCuFeS2ЕШдгжЪ)жЦБИИпДПЖўСђЛЏютЕФвЛжжЩњВњЙЄвеШчЯТЃК

ЛиД№ЯТСаЮЪЬтЃК
(1)ЁАЫсНўЁБжаМгШыЧтЗњЫсЪЧЮЊСЫГ§ШЅдгжЪSiO2ЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________ЁЃ
(2)дкЁАбѕЛЏБКЩеЁБЙ§ГЬжажївЊЪЧНЋMoS2зЊЛЏЮЊMoO3ЃЌдкИУЗДгІжабѕЛЏМСгыЛЙдМСЕФЮяжЪЕФСПжЎБШЮЊ________ЁЃ
(3)ШєбѕЛЏБКЩеВњЮяВњЩњЩеНсЯжЯѓЃЌдкЁААБНўЁБЧАЛЙашНјааЗлЫщДІРэЃЌЦфФПЕФЪЧ_________ЃЌЁААБНўЁБКѓЩњГЩ( NH4)2 MoO4ЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________ЁЃ
(4)ЯђЁААБНўЁБКѓЕФТЫвКжаМгШыNa2SКѓЃЌютЫсяЇзЊЛЏЮЊСђДњютЫсяЇ[(NH4)2MoS4]ЃЌМгШыбЮЫсКѓЃЌ(NH4)2 MoS4гыбЮЫсЗДгІЩњГЩMoS3ГСЕэЃЌГСЕэЗДгІЕФРызгЗНГЬЪНЮЊ_________________ЁЃ
(5)ИпДПMoS2жаШдШЛЛсДцдкМЋЮЂСПЕФЗЧећБШОЇЬхMoS2.8ЕШдгжЪЃЌдкИУдгжЪжаЮЊБЃГжЕчжаадЃЌMoдЊЫига+4ЁЂ+6СНжжМлЬЌЃЌдђMoS2жаMo4+ЫљеМMoдЊЫиЕФЮяжЪЕФСПЗжЪ§ ЮЊ__________ЁЃ
ЮЊ__________ЁЃ
(6)ютЫсФЦОЇЬх( Na2 MoO4 2H2O)ЪЧвЛжжЮоЙЋКІаЭРфШДЫЎЯЕЭГН№ЪєЛКЪДМСЃЌПЩвдгЩMoS2жЦБИЁЃдкжЦБИЙ§ГЬжаашМгШыBa(OH)2ЙЬЬхГ§ШЅSO42-ЃЌШєШмвКжаc(MoO42-)=0.4 mol/LЃЌc(SO42-)=0. 05 mol/LЃЌГЃЮТЯТЃЌЕБBaMoO4МДНЋПЊЪМГСЕэЪБЃЌSO42-ЕФШЅГ§ТЪЮЊ____________ [КіТдШмвКЬхЛ§БфЛЏЁЃвбжЊЃК259ЁцЃЌKsp( BaMoO4)=4.0ЁС10-8 ЃЌ Ksp(BaSO4)=1.1ЁС10-10]ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗДгІA+BЁњC ЁїH ЃМ0ЃЌЗжСНВННјаа Ђй A+BЁњX ЁїHЃО0 Ђк XЁњC ЁїHЃМ0 ЁЃЯТСаЪОвтЭМжаЃЌФме§ШЗБэЪОзмЗДгІЙ§ГЬжаФмСПБфЛЏЕФЪЧ
A.  B.
B. 
C.  D.
D. 
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗХШШЗДгІCOЃЋH2O(g)![]() CO2 ЃЋH2 дкЮТЖШt1ЪБДяЕНЦНКтЃЌc1 (CO)ЃНc1 (H2O)ЃН1.0 molЁЄLЃ1ЃЌЦфЦНКтГЃЪ§ЮЊK1ЁЃЩ§ИпЗДгІЬхЯЕЕФЮТЖШжСt2ЪБЃЌЗДгІЮяЕФЦНКтХЈЖШЗжБ№ЮЊc2 (CO)КЭc2 (H2O)ЃЌЦНКтГЃЪ§ЮЊK2ЃЌдђ
CO2 ЃЋH2 дкЮТЖШt1ЪБДяЕНЦНКтЃЌc1 (CO)ЃНc1 (H2O)ЃН1.0 molЁЄLЃ1ЃЌЦфЦНКтГЃЪ§ЮЊK1ЁЃЩ§ИпЗДгІЬхЯЕЕФЮТЖШжСt2ЪБЃЌЗДгІЮяЕФЦНКтХЈЖШЗжБ№ЮЊc2 (CO)КЭc2 (H2O)ЃЌЦНКтГЃЪ§ЮЊK2ЃЌдђ
A.K2КЭK1ЕФЕЅЮЛОљЮЊmolЁЄLЃ1B.c2(CO)>c2(H2O)C.K2<K1D.c1(CO)>c2(CO)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЁЂEЁЂFЁЂGЁЂHЪЧЯрЖдЗжзгжЪСПвРДЮдіДѓЕФЦјЬхЃЌЫќУЧОљгЩЖЬжмЦкдЊЫизщГЩЃЌОпгаШчЯТаджЪЃК
ЂйBФмЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЃЌAЁЂCЁЂDВЛФмЪЙЪЊШѓЕФЪЏШяЪджНБфЩЋЃЌEЁЂGОљПЩЪЙЪЊШѓЕФРЖЩЋЪЏШяЪджНБфКьЃЛ
ЂкFГЪКьзиЩЋЃЛ
ЂлGКЭHОљФмЪЙЦЗКьЭЪЩЋЃЌAдкHжаАВОВШМЩеВЂВњЩњВдАзЩЋЛ№бцЃЛ
ЂмCдкDжаЭъШЋШМЩеЩњГЩEКЭH2OЃЌЭЌЪБЗХГіДѓСПШШЃЌЙЄвЕЩЯПЩРћгУИУЗДгІКИНгЛђЧаИюН№ЪєЁЃ
ЧыЛиД№ЯТСаЮЪЬтЃК
(1)EЕФЕчзгЪНЮЊ_____ЃЌDжаЫљКЌдЊЫиЕФЛљЬЌдзгКЫЭтЕчзгХХВМЪНЮЊ___ЃЌCЗжзгжаЕФІвМќКЭІаМќЕФИіЪ§жЎБШЮЊ___ЁЃ
(2)аДГіЪЕбщЪвгУЙЬЬхвЉЦЗжЦШЁBЕФЛЏбЇЗНГЬЪН_______________ЁЃ
(3)ШєДгaПкЭЈШыЦјЬхGЃЌДгbПкЭЈШыЦјЬхFЃЌXЮЊТШЛЏБЕШмвКЃЌЙлВьЕНЕФЯжЯѓЪЧ_____________,
ЗДгІЕФРызгЗНГЬЪНЮЊ_________________ЁЃ

(4)вбжЊЃКE(g)+3A(g)![]() CH3OH(l)+H2O(l) ІЄH=-53.66 kJЁЄmol-1
CH3OH(l)+H2O(l) ІЄH=-53.66 kJЁЄmol-1
2CH3OH(l)![]() CH3OCH3(g)+H2O(l) ІЄH=-23.4 kJЁЄmol-1
CH3OCH3(g)+H2O(l) ІЄH=-23.4 kJЁЄmol-1
аДГіEгаДпЛЏМСЪБгыAКЯГЩЖўМзУб(CH3OCH3)ЕФШШЛЏбЇЗНГЬЪН_____________ЁЃ
(5)ЦјЬхCФмЪЙСђЫсЫсЛЏЕФИпУЬЫсМиШмвКЭЪЩЋЃЌВњЮяжЎвЛЪЧEЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com