
����Ŀ��ʵ��������500mL0.1mol/LNa2CO3����Һ�ش���������
��1������Na2CO3��Һʱ���õ���Ҫ������������ƽ����ֽ���ձ���ҩ�ס���Ͳ��___��___��___��
��2��������Һ�������£�
A.��������ƽ��ȡNa2CO3��10H2O����___g��
B.�����������ձ��У�����Ͳ�����м�ˮ�ܽ⣬���ָ������£�
C.�ò���������������Һת��������ƿ��
D.ϴ�Ӳ��������ձ�2-3�Σ���ϴ��Һ��ת��������ƿ�У�
E.___��
F.����������ƿ��עˮ����Һ��������ƿ���̶�����___cmʱ������___�μ�����ˮ��Һ����̶������У�
G.�Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת���ҡ�ȣ�
H.����Һת�����Լ�ƿ�У����ϱ�ǩ����ע�Լ����ơ�Ũ�ȼ�����ʱ�䡣
��3����ʵ���������������Һ��Ũ����ƫ�ߣ�ƫ�ͻ��Dz��䣿
A.��ˮʱ�����̶���___��
B.�ܽ��δ��ȴ�����¾�ת������ƿ___��
C.����ƿ�ڱڸ���ˮ���δ���ﴦ��___��
D.����ʱ����___��
E.���µߵ�ҡ�Ⱥ�Һ����ڿ̶���___��
���𰸡�500mL����ƿ ������ ��ͷ�ι� 14.3g ����ҡ������ƿ��ʹ��Һ��Ͼ��� 1-2 ��ͷ�ι� ƫ�� ƫ�� ���� ƫ�� ����
��������
��1������500mL0.1mol/LNa2CO3��Һʱ���õ���Ҫ������������ƽ����ֽ���ձ���ҩ�ס���Ͳ��500mL����ƿ������������ͷ�ιܣ�
��Ϊ��500mL����ƿ������������ͷ�ιܣ�
��2��������Һ�������£�
A. 500mL0.1mol/LNa2CO3��Һ������Na2CO3�����ʵ���=0.5L��0.1mol/L=0.05mol����0.05mol̼���Ƶ�Na2CO3��10H2O������=0.05mol��286g/mol=14.3g������������ƽ��ȡNa2CO3��10H2O����14.3g��
E. ����ҡ������ƿ��ʹ��Һ��Ͼ��ȣ�
F.����������ƿ��עˮ����Һ��������ƿ���̶�����1-2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ����̶������У�
��Ϊ��14.3g������ҡ������ƿ��ʹ��Һ��Ͼ��ȣ�1-2����ͷ�ιܣ�
��3��A.��ˮʱ�����̶�������Һ�������ƫ����c=![]() ����ҺŨ��ƫ�ͣ�
����ҺŨ��ƫ�ͣ�
B.�ܽ��δ��ȴ�����¾�ת������ƿ������Һ�ָ������£���Һ�������С������c=![]() ����ҺŨ��ƫ�ߣ�
����ҺŨ��ƫ�ߣ�
C.����ƿ�ڱڸ���ˮ���δ���ﴦ������Ӱ����Һ��Ũ�ȴ�С�����Ƶ���ҺŨ�Ȳ��䣻
D.����ʱ���ӣ�������Һ�����ƫ����c=![]() ����ҺŨ��ƫ�ͣ�
����ҺŨ��ƫ�ͣ�
E.���µߵ�ҡ�Ⱥ�Һ����ڿ̶��ߣ����ڿ̶������ϲ�����Һ�Σ�����һ��ʱ�䣬Һ���ָ����̶��ߣ�����Һ�ܶ���Ӱ�죬��ҺŨ�Ȳ��䣻
��Ϊ��ƫ�ͣ�ƫ�ߣ����䣻ƫ�ͣ����䡣


 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�� (1)һ������ѱ�����ΪNa2O���ƿ�10.8 g������Ͷ��100 mLˮ�У�������״����2.24 L����(������Һ����仯�ɺ���)��������ƿ������������Ƶ�����������_________(����С�����һλ)��������Һ�����ʵ����ʵ���Ũ��Ϊ_________��
(2)��֪ʵ����Ҳ����KMnO4��Ũ���ᷴӦ�Ʊ���������ѧ����ʽΪ2KMnO4+16HCl��2KCl+5Cl2��+2MnCl2+8H2O������˫���ŷ���ʾ������ת�Ƶķ������Ŀ______________���÷�Ӧ�л�ԭ����Ϊ______________��
(3)̼�������׳�С�մ���д��̼�����Ƶĵ��뷽��ʽ_______________________��̼�����Ƽ������ᷴӦ������Ӧ����д��̼��������Һ��NaOH��Һ��Ӧ�����ӷ���ʽ
_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��������Ĺ�������ʾ��ͼ���£�

���������գ�
��1������ˮ���������A��B�����ʣ�������A��Դ��ʯ��Ҥ������д��A��B�Ļ�ѧʽ��
A__________________________________B__________________________________
��2��ʵ�����ᴿ���ε�ʵ���������Ϊ��
ȡ����______��������______��______����ȴ�ᾧ��______����ɡ�
��3����ҵ��������������У�̼�ữʱ������������____________________________��
̼�ữʱû������̼���ƾ��壬��ԭ����_______________________________________��
��4��̼�ữ����ˣ���ҺD����Ҫ�ijɷ���_____________________________����д��ѧʽ����������һ�ɷֵ������ӵľ��巽���ǣ�________________________________________��
��5����������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ��ˮ����������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��_________________________________________________________
��ҺD��ʯ��ˮǰ��Ҫ���ȣ�ԭ����_____________________________________________��
��6����Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ��
_____________________________________________________________________________
��ע����ı���ʽ�����õ��йط��ŵĺ��壩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ���Ҳ�����ȷ���ǣ� ��
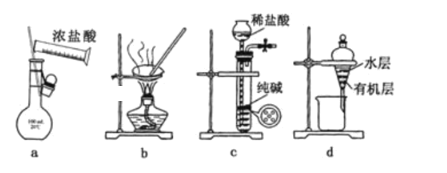
A.��ͼa��ʾװ������l00mL0.100mo1L-1ϡ����
B.��ͼb��ʾװ������NaCl��Һ�Ʊ�NaCl����
C.��ͼc��ʾװ����ȡ����CO2����
D.��ͼd��ʾװ�÷����ñ���ȡ��ˮ���ѷֲ���л����ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������10 mL 0.1 mol��L��1 NaOH��Һ�м���0.1 mol��L��1��һԪ
��HA����ҺpH�ı仯������ͼ��ʾ������˵����ȷ����

A. a����ʾ��Һ��c(Na��)��c(A��)��c(H��)��c(HA)
B. a��b������ʾ��Һ��ˮ�ĵ���̶���ͬ
C. pH��7ʱ��c(Na��)��c(A��)��c(HA)
D. b����ʾ��Һ��c(A��)��c(HA)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������ԭ��Ϊ0.2��6.02��1024����NH4HCO3����������___�����к���ԭ�ӵ����ʵ���Ϊ___��3.6��ˮ�к���������Ϊ___������٤������ΪNA����
��2����ʵ����Ҫ��12mol��L-1����������Һ������Ũ��Ϊ0.6mol��L-1��ϡ����������Һ100mL������Ҫ����Ũ��������___mL����Ҫ��������ƽ����___���������Ʋ������Ƴ�0.5mol��L-1������������Һ100mL��
��3���������aLHCl�����������Ƴ�1L������Һ��������Һ�ܶ�Ϊdg/cm3����������Һ���ʵ���Ũ��Ϊ___mol/L������Һ�����ʵ���������Ϊ___��
��4���ڱ�״���£�ij����A���ܶ���1.25g��L-1��������Ħ��������___��ͬ��ͬѹ�¸�����������������ܶ���___���ڱ�״���£���CO��CO2��ɵĻ������Ϊ6.72L������Ϊ12g���˻�������ƽ����Է���������___���������CO��CO2���ʵ���֮����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з���ʽ��д��ȷ����
A. HCO3����ˮ��Һ�еĵ��뷽��ʽ��HCO3����H2O![]() H3O����CO32��
H3O����CO32��
B. H2SO4�ĵ��뷽��ʽH2SO4![]() 2H����SO42��
2H����SO42��
C. CO32����ˮ�ⷽ��ʽ��CO32����2H2O![]() H2CO3��2OH��
H2CO3��2OH��
D. CaCO3�ĵ��뷽��ʽ��CaCO3![]() Ca2+��CO32��
Ca2+��CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���� 0.1mo1��L-1 �� H2SO4 ��Һ�е��� 0.1mo1��L-1 ������ Ba(OH)2 ��Һ����Һ�ĵ������������仯�������ǿ�� (I) ����� Ba(OH)2 ��Һ����� (V) �ı仯������ȷ����
A.  B.
B. 
C.  D.
D. 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ȼ�ϵ��ԭ���о������°��ĺϳɣ���ع���ʱMV2+/MV+�ڵ缫��ø֮�䴫�ݵ��ӣ�ʾ��ͼ������ʾ������˵���������
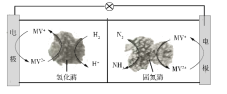
A. ������й�ҵ�ϳɰ����÷��������ºͣ�ͬʱ�����ṩ����
B. �����������⻯ø�����·�����ӦH2+2MV2+![]() 2H++2MV+
2H++2MV+
C. ���������̵�øΪ������N2������ԭ��Ӧ����NH3
D. ��ع���ʱ����ͨ������Ĥ�ɸ��������������ƶ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com