
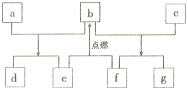
����Ŀ��������Ԫ��W��X��Y��Z��ԭ�������������ӣ�����ЩԪ����ɵij������ʵ�ת����ϵ����ͼ,����a��b��d��gΪ�����aΪ����ɫ���壬c��Z�ĵ��ʣ������ȷ�Ӧ�г�����������e��fΪ�������嵥�ʡ������й�˵����ȷ����
A. �����ӵİ뾶��Y>Z>X
B. ���⻯��ķе㣺Y>X
C. ����������Ӧˮ����ļ��ԣ�Z>Y
D. W��Y��������������ѧ��������ͬ
���𰸡�B
��������������������Ԫ��W��X��Y��Z��ԭ�������������ӣ�����a��b��d��gΪ�����aΪ����ɫ���壬��aΪ�������ƣ�c��Z�ĵ��ʣ������ȷ�Ӧ�г�����������cΪ����þ��e��fΪ�������嵥�ʣ����ݿ�ͼ��þ�뻯����b��Ӧ�������嵥��f����fΪ���������������뻯����b��Ӧ�������嵥��e����eΪ��������bΪˮ��dΪ�������ƣ�gΪ������þ��
��⣺��������������aΪ�������ƣ�bΪˮ��cΪþ��dΪ�������ƣ�eΪ������fΪ������gΪ������þ����WΪH��XΪO��YΪNa��ZΪMg��A. XΪO��YΪNa��ZΪMg�������Ӿ�����ͬ�ĵ��Ӳ�ṹ�����Ӱ뾶��X>Y>Z����A����B. �⻯�������ӻ�����е����ˮ����B��ȷ��C.Ԫ�صĽ�����Խǿ������������Ӧˮ����ļ���Խǿ�����ԣ�Y>Z����C����D.ˮ��������ⶼ�ǹ��ۻ����ֻ���й��ۼ��������ƻ�������ƶ��������ӻ�����������Ӽ�����ѧ�����Ͳ�ͬ����D����ѡB��


 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������ѧ�仯����
A.ȼ��Һ������Һ��ȼB.�Ϻ�����ˮ���Ժ���
C.��ɽ��Ҷ��Ҷ��ɽ��D.��ɽ��Ҷ��Ҷ��ɽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС�����������װ����ȡ����֤SO2�����ʡ�

��ش���
��1��д��ͼ��������������a___________��b___________��
��2��������NaOH��Һ��������______________________��
��3��Ϊ����֤SO2�����������ϴ��ƿ���п�ѡ����Լ���_____________________��
A.��ɫʯ����Һ B.��ɫ��̪��Һ C.����ʯ��ˮ D.��ˮ
��4������˵����ȷ����____________��
A.ʵ�鿪ʼʱ��ֻ���Һ©��������������ʹҺ��˳������
B.����װ���м����Լ�(ҩƷ)���ٽ��������Լ��
C.ʵ�鿪ʼ��ϴ��ƿ���͢�ֻ��Һ����ɫ�����߾���֤��SO2����Ư����
D.ʵ�鿪ʼ��ϴ��ƿ���пɹ۲쵽��ɫ�����������������˵��SO2���л�ԭ��
��5��ϴ��ƿ���з�����Ӧ�����ӷ���ʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ȵ���ø������һ������ij������(����)���¶ȱ���37 ����pH����������ֵ�����������뷴Ӧʱ���ϵ����ͼ����ش��������⡣

(1)��ø���õĵ����ǡ�����������
(2)��140 min�����߱��ˮƽ��������Ϊ
________________________________________________________________________��
(3)�������ȵ���øŨ�ȣ������������䣬����ԭͼ�ϻ������������仯��ʾ�����ߡ�
(4)���ȵ���øŨ�Ⱥ������������䣬��ӦҺpH��2�����ߵ�10����ø����Ӧ�����ʽ�________��ԭ����____________________________________��
(5)��ͼ������ȷ��ʾ�ȵ���ø�Ե���ķֽ����ʺ��¶ȹ�ϵ����( )
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ͬ��ѧԪ������ɵĻ��������˵������ȷ����

A. ��ͼ�Т�Ϊij�ֶ����ĵ��壬���������ǰ�����
B. ���ڴ�����Ƥ�º�����������Χ�Ȳ�λ�������֬��
C. ��һ���Ǻ��������ɺ���ĵ��������
D. �����Dz��빹��ֲ��ϸ���ڵ�һ�ֶ��ǣ�������������ά��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ɫ��Һ�д��������һ�������ǣ� ��
A.NH4+��SO42-��OH-��K+B.Fe3+��NO3-��Cl-��H+
C.K+��HCO3-��Cl-��H+D.H+��Na+��Cl-��SO42-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������и������ʰ����ʡ��ᡢ��η���˳�����У�������ȷ���ǣ� ��
A.ˮ�������ᡢ�ռ��������B.��ˮ�����ᡢ�����ᱵ
C.���������ᡢ�������D.ͭ�����ᡢʯ��ʯ���Ȼ�ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�²�ϸ�����ڵ��ⶾ�أ���ɫ��ϸ��״�ᾧ����С��������к�ǿ�Ķ��ԣ����������ѡ�Ż�¡���Ѫ�����εȣ��������������ⶾ��Ϊ��״�ģ��ṹʽ��ͼ��ʾ�����ͼ�����ش�

��1���û������к�������İ���_____________�����Ȼ�________________����
��2���û���������_____________����������ɵģ�������Щ�������������������ṹ�е�_____________��
��3����ɸû�����İ�������___________�֣�������_____________���������R����ͬ�����R����_______________��
��4���û������Ϊ��״__________�Ļ��������___________���ļ���
��5����д���߿��ڽṹ�����ƣ�A._________________��B.___________________��
��6���û��������8����ԭ�ӣ�����_____________��λ���ļ��ϣ�____________��λ��R���ϡ�
��7�����ⶾ�ػ������γɹ�����ʧȥ��______________��ˮ���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������Ǵ�����Ⱦ��֮һ���û���̿��һ����̼��ԭ��������ɷ�ֹ������Ⱦ���ش��������⣺
��֪��2C(s)+O2(g)=2CO(g) ��H=- 221 kJ/mol
C(s)+O2(g)=CO2(g) ��H=- 393.5 kJ/mol
N2(g)+O2(g)=2NO(g) ��H= +181 kJ/mol
��1����ij��Ӧ��ƽ�ⳣ������ʽΪK=![]() ����д���˷�Ӧ���Ȼ�ѧ����ʽ��___________________________�����д�ʩ�ܹ�����˷�Ӧ��NO��ת���ʵ���(����ĸ����)_________��
����д���˷�Ӧ���Ȼ�ѧ����ʽ��___________________________�����д�ʩ�ܹ�����˷�Ӧ��NO��ת���ʵ���(����ĸ����)_________��
a.��������ѹǿ b.�����¶� c.ʹ�����ʴ��� d.����CO��Ũ��
��2�����ݻ�Ϊ2L���ܱ������м������̿(����)��NO��������ӦC(S)+2NO(g)![]() N2(g)+CO2(g)��NO��N2�����ʵ����仯���±���ʾ��
N2(g)+CO2(g)��NO��N2�����ʵ����仯���±���ʾ��
���ʵ���/mol | T1/�� | T2/�� | |||||
0 | 5 min | 10 min | 15 min | 20 min | 15 min | 30 min | |
NO | 2.0 | 1.16 | 0.80 | 0.80 | 0.50 | 0.40 | 0.40 |
N2 | 0 | 0.42 | 0.60 | 0.60 | 0.75 | 0.80 | 0.80 |
��0��5min�ڣ���CO2��ʾ�ĸ÷�Ӧ����v(CO2)=______���������µ�ƽ�ⳣ��K=_______________��
�ڵ�15 min���¶ȵ�����T2�����ݱ仯���ϱ���ʾ����T1_______ T2(�>������<����=��)��
����30minʱ������T2���䣬����������ټ�������ַ�Ӧ������2 mol�����ʱ��Ӧ_______�ƶ�(�������������)�����մ�ƽ��ʱNO��ת����a=______________��
��3����ҵ�Ͽ����ð�ˮ��ȥ��ӦC(s)+2NO(g)![]() N2(g)+CO2(g)�в�����CO2���õ�NH4HCO3��Һ����ӦNH4++HCO3-+H2O
N2(g)+CO2(g)�в�����CO2���õ�NH4HCO3��Һ����ӦNH4++HCO3-+H2O![]() NH3��H2O+H2CO3��ƽ�ⳣ��K=________________��(��֪������NH3��H2O�ĵ���ƽ�ⳣ��Kb=2��10-5�� H2CO3�ĵ���ƽ�ⳣ��Ka1=4��10-7��Ka2=4��10-11)
NH3��H2O+H2CO3��ƽ�ⳣ��K=________________��(��֪������NH3��H2O�ĵ���ƽ�ⳣ��Kb=2��10-5�� H2CO3�ĵ���ƽ�ⳣ��Ka1=4��10-7��Ka2=4��10-11)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com