
ТСЦЄCl2єНјоИЬТєФЪІ»Н¬МхјюПВЈ¬µГµЅµДІъОпІ»Н¬ЎЈДіРЛИ¤РЎЧйУГПВНјЛщКѕЧ°ЦГЦЖИЎВИЛбјШЎўґОВИЛбДЖєНМЅѕїВИЛ®µДРФЦКЎЈЈЁ3Cl2+6KOH KClO3+5KCl+3H2O Ј©
KClO3+5KCl+3H2O Ј©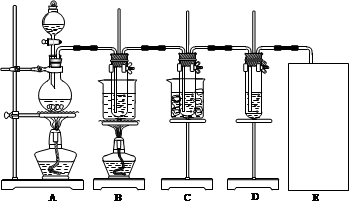
НјЦРЈєAОЄВИЖш·ўЙъЧ°ЦГЈ»BµДКФ№ЬАпКўУР15 mL 30% KOHИЬТєЈ¬ІўЦГУЪЛ®ФЎЦРЈ»CµДКФ№ЬАпКўУР15 mL 8% NaOHИЬТєЈ¬ІўЦГУЪ±щЛ®ФЎЦРЈ»DµДКФ№ЬАпјУУРЧПЙ«КЇИпКФТєЎЈЗлМоРґПВБРїХ°ЧЈє
ЈЁ1Ј©ЦЖИЎВИЖшК±Ј¬ФЪФІµЧЙХЖїАпјУИлТ»¶ЁЦКБїµД¶юСх»ЇГМЈ¬НЁ№э (МоТЗЖчГыіЖ)ПтФІµЧЙХЖїЦРјУИлККБїµДЕЁСОЛбЎЈЧ°ЦГAЦР·ґУ¦µДАлЧУ·ЅіМКЅОЄ ЎЈРиТЄПтЙХЖїЦРјУЛйґЙЖ¬ВрЈї ЈЁСЎМоЈєЎ°РиТЄЎ±ЎўЎ°І»РиТЄЎ±Ј©ЎЈ
ЈЁ2Ј©·ґУ¦ЅбКшєуЈ¬ЙХЖїАпµДИЬТє ЎЈ
AТ»¶ЁПФЛбРФЈ»BїЙДЬПФЛбРФЈ¬ТІїЙДЬОЄЦРРФЈ»CЦ»УРСх»ЇРФЈ»DЦ»УР»№ФРФЈ»EјИУРСх»ЇРФУЦУР»№ФРФ
ЈЁ3Ј©·ґУ¦Нк±ПЈ¬BКФ№ЬЦРУРЙЩБїѕ§МеОціцЈ¬ѕАдИґєуЈ¬УРґуБїѕ§МеОціцЎЈНјЦР·ыєПёГѕ§МеИЬЅв¶ИЗъПЯµДКЗ (Мо±аєЕЧЦДё)Ј»ґУBµДКФ№ЬЦР·ЦАліцёГѕ§Ме±ШРлУГµЅµДІЈБ§ТЗЖчУР ЎЈ
ЈЁ4Ј©ёГРЎЧйН¬С§·ўПЦЦЖµГµДВИЛбјШІъБїЖ«µНЈ¬їЙДЬµДТ»ЦЦФТтКЗCl2ЦРє¬УРHClЖшМеЎЈ¶ФґЛОКМвїЙТФНЁ№эёДЅшКµСйЧ°ЦГµД·Ѕ·ЁЅшРР±ЬГвЎЈ·Ѕ·ЁКЗ ЎЈ
ЈЁ5Ј©КµСйЦРїЙ№ЫІмµЅDµДКФ№ЬАпИЬТєµДСХЙ«УЙЧПЙ«ПИ±дОЄ_________Й«Ј¬ЧоЦХ±дОЄ________Й«ЎЈ
ЈЁ6Ј©CЧ°ЦГЦР·ґУ¦Нк±ПµДПЦПуКЗ______________________________________________ЎЈ
ЈЁ7Ј©ЗлФЪЧ°ЦГНј·ЅїтЦР»іцИ±ЙЩµДКµСйЧ°ЦГЈ¬ІўЧўГчКФјБЎЈ
·ЦТєВ©¶·ЈЁ1·ЦЈ©Ј»MnO2+4H++2Cl- Mn2++Cl2Ўь+2H2OЈЁ2·ЦЈ©ЈЁ»ЇС§КЅґнОуЎўОґЕдЖЅОЮ·ЦЎЈµфЎўґн·ґУ¦МхјюЈ»µфЎўґн№ШјьјэН·Ј»µИєЕУГіЙјэН·»тїЙДж·ыєЕЈ»єПјЖїЫ1·ЦЎЈґЛ±кЧјПВН¬Ј©ЎЈІ»РиТЄЈЁ1·ЦЈ©ЈЁЛщУРСЎМоЈ¬±ШРлЧсКШКФМвЛµ·ЁЈ¬·сФтІ»ёш·ЦЈ¬АэИз±ѕїХМоЎ°І»Ў±І»ёш·ЦЎЈґЛ±кЧјПВН¬Ј©
Mn2++Cl2Ўь+2H2OЈЁ2·ЦЈ©ЈЁ»ЇС§КЅґнОуЎўОґЕдЖЅОЮ·ЦЎЈµфЎўґн·ґУ¦МхјюЈ»µфЎўґн№ШјьјэН·Ј»µИєЕУГіЙјэН·»тїЙДж·ыєЕЈ»єПјЖїЫ1·ЦЎЈґЛ±кЧјПВН¬Ј©ЎЈІ»РиТЄЈЁ1·ЦЈ©ЈЁЛщУРСЎМоЈ¬±ШРлЧсКШКФМвЛµ·ЁЈ¬·сФтІ»ёш·ЦЈ¬АэИз±ѕїХМоЎ°І»Ў±І»ёш·ЦЎЈґЛ±кЧјПВН¬Ј©
ЈЁ2Ј©AEЈЁ2·ЦЈ©ЈЁИ«¶ФµГ2·ЦЈ¬¶Ф¶шІ»И«µГ1·ЦЈ¬ЖдУаІ»µГ·ЦЈ©
ЈЁ3Ј©MЈЁ1·ЦЈ©Ј»В©¶·ЈЁ»тЖХНЁВ©¶·ЎўИэЅЗВ©¶·Ј©ЎўІЈБ§°фЈЁІ»ДЬРґОЄІЈ°фЈ©ЎўЙХ±ЈЁ»тРЎЙХ±Ј©ЈЁ1·ЦЈ© ЈЁИ«¶ФІЕДЬµГ1·ЦЈ©
ЈЁ4Ј©ФЪAЎўBЧ°ЦГЦ®јдјУТ»ёцКў±ҐєНКіСОЛ®µДКФ№ЬЈЁ»тПґЖшЖїЎўКФјБЖїЈ©ЈЁ2·ЦЈ©ЈЁО»ЦГ1·ЦЎўКФјБ1·ЦЎЈ¶АБўІЙ·ЦЎЈДіµгґнЈ¬І»У°ПмБнТ»µгµГ·ЦЎЈО»ЦГЈєAєу»тBЗ°І»µГ·ЦЎЈКФјБЈє±ҐєН±ШРлУРЈ¬NaClїЙТФКЗЖдЛьИЬЅв¶ИЅПґуµДСОЛбСОЎЈЈ©
ЈЁ5Ј©ємЈЁ1·ЦЈ©Ј»ОЮЈЁ1·ЦЈ© ЈЁ6Ј©CЦРКФ№ЬЙПІїїХјдідВъ»ЖВМЙ«ЖшМеЈЁ1·ЦЈ¬ЖдЛыєПАнґр°ёёш·ЦЈ©
ЈЁ7Ј©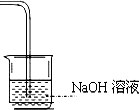 ўЩКФјБОЄЗїјоИЬТєЈЁЕЁ¶ИІ»ЧчТЄЗуЈ©ЈЁ1·ЦЈ©ЎЈўЪµј№ЬТЄНЁИлТєГжЦБЙЩ1/3Ј¬М«ЗіІ»їЙ ўЫ КЗТ»ёціЁїЪМеПµЎЈЈЁ1·ЦЈ©ЈЁўЩєНўЪўЫ·ЦїЄІЙ·ЦЈ¬±ЛґЛІ»ЗЈБ¬ЎЈўЪўЫґнТ»МхЈ¬ФтµЪ2·ЦІ»ёшЎЈЈ©
ўЩКФјБОЄЗїјоИЬТєЈЁЕЁ¶ИІ»ЧчТЄЗуЈ©ЈЁ1·ЦЈ©ЎЈўЪµј№ЬТЄНЁИлТєГжЦБЙЩ1/3Ј¬М«ЗіІ»їЙ ўЫ КЗТ»ёціЁїЪМеПµЎЈЈЁ1·ЦЈ©ЈЁўЩєНўЪўЫ·ЦїЄІЙ·ЦЈ¬±ЛґЛІ»ЗЈБ¬ЎЈўЪўЫґнТ»МхЈ¬ФтµЪ2·ЦІ»ёшЎЈЈ©
ЅвОцКФМв·ЦОцЈєЈЁ1Ј©ПтЙХЖїЦРјУИИЕЁСОЛбЈ¬РиТЄЅиЦъУЪ·ЦТєВ©¶·ЎЈФЪјУИИµДМхјюПВ¶юСх»ЇГМСх»ЇЕЁСОЛбЙъіЙВИЖшЈ¬·ґУ¦µДАлЧУ·ЅіМКЅОЄMnO2+4H++2Cl- Mn2++Cl2Ўь+2H2OЈ»УЙУЪФЪ·ґУ¦ЦР¶юСх»ЇГМІ»ИЬУЪЛ®Ј¬ТФ№ММеµДРОКЅґжФЪЈ¬ТтґЛ·ґУ¦ЦРІ»РиТЄФЩјУИИЛйґЙЖ¬ЎЈ
Mn2++Cl2Ўь+2H2OЈ»УЙУЪФЪ·ґУ¦ЦР¶юСх»ЇГМІ»ИЬУЪЛ®Ј¬ТФ№ММеµДРОКЅґжФЪЈ¬ТтґЛ·ґУ¦ЦРІ»РиТЄФЩјУИИЛйґЙЖ¬ЎЈ
ЈЁ2Ј©УЙУЪФЪ·ґУ¦№эіМЦРЕЁСОЛбµДЕЁ¶ИЦрЅҐЅµµНЈ¬¶ш¶юСх»ЇГМІ»ДЬСх»ЇПЎСОЛбЈ¬ЛщТФ·ґУ¦ЅбКшєуСОЛбТ»¶ЁКЈУаЈ¬ИЬТєПФЛбРФЎЈН¬К±·ґУ¦ЦР»№ЙъіЙВИ»ЇГМѕЯУР»№ФРФЎЈ¶шИЬТєЦРµДЗвАлЧУ»№ѕЯУРСх»ЇРФЈ¬ЛщТФґр°ёСЎAEЎЈ
ЈЁ3Ј©АдИґєуУРґуБїѕ§МеОціцЈ¬ХвЛµГчёГОпЦКµДИЬЅв¶ИКЬОВ¶ИµДУ°ПмЅПґуЎЈЗТИЬЅв¶ИЛжОВ¶ИµДЙэёЯ¶шФцґуЈ¬ЛщТФ·ыєПМхјюµДЗъПЯКЗMЎЈґУИЬТєЦР·ЦАліц№ММеµДІЩЧчКЗ№эВЛЈ¬РиТЄµДІЈБ§ТЗЖчУРВ©¶·ЎўІЈБ§°фЎўЙХ±ЎЈ
ЈЁ4Ј©УЙУЪВИ»ЇЗвј«ТЧИЬУЪЛ®Ј¬ВИЖшФЪЛ®ЦРµДИЬЅв¶ИЅПРЎЈ¬ЛщТФТЄіэИҐВИЖшЦРµДВИ»ЇЗвЖшМеЈ¬їЙТФНЁИлµЅКўУР±ҐєНКіСОЛ®µДПґЖшЖїЦРЈ¬јґХэИ·Чц·ЁКЗФЪAЎўBЧ°ЦГЦ®јдјУТ»ёцКў±ҐєНКіСОЛ®µДПґЖшЖїЎЈ
ЈЁ5Ј©ВИЖшИЬУЪЛ®ЙъіЙСОЛбУлґОВИЛбЎЈИЬТєПФЛбРФЈ¬ФтКЇИпКФТє±дОЄємЙ«ЎЈУЦТтОЄґОВИЛб»№ѕЯУРЗїСх»ЇРФЈ¬ДЬЖЇ°ЧЛбјоЦёКѕјБЈ¬ТтґЛИЬТєЧоЦХ±дОЄОЮЙ«ЎЈ
ЈЁ6Ј©ФЪ±щЛ®ФЎЦРВИЖшУлЗвСх»ЇДЖИЬТєµД·ґУ¦єЬВэЈ¬ЗТВИЖшФЪЛ®ЦРµДИЬЅв¶ИєЬРЎЈ¬ЛщТФCЧ°ЦГЦР·ґУ¦Нк±ПµДПЦПуКЗCЦРКФ№ЬЙПІїїХјдідВъ»ЖВМЙ«ЖшМеЎЈ
ЈЁ7Ј©ВИЖшУР¶ѕЈ¬РиТЄОІЖшґ¦АнЈ¬Т»°гУГЗвСх»ЇДЖИЬТєОьКХЈ¬ФтКµСйЧ°ЦГНјОЄ ЎЈ
ЎЈ
їјµгЈєїјІйВИЖшЦЖ±ёЎўѕ»»ЇЎўОІЖшґ¦АнТФј°РФЦККµСйМЅѕї



| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
ёщѕЭПВНјЧ°ЦГЅшРРКµСйЈ¬ТСЦЄЈєNa2O2УлH2OєНCO2¶јДЬ·ґУ¦ІўЙъіЙO2,µ«УлNH3І»·ґУ¦
»ШґрПВБРОКМвЈєЎЈ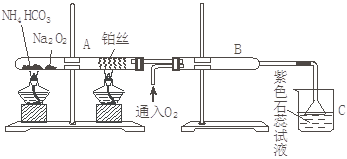
ЈЁ1Ј©ФЪКЬИИµДКФ№ЬAЦРNH4HCO3·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅОЄЈє ЎЈ
ЈЁ2Ј©±»јУИИµДІ¬Лїґ¦·ўЙъµД»ЇС§·ЅіМКЅОЄЈє___________________________________ЎЈ
ЈЁ3Ј©BЦРіцПЦµДПЦПуОЄЈє___________________________________________________ЎЈ
ЈЁ4Ј©ЙХ±CЦР·ўЙъµДПЦПуОЄ________________________________________________ЎЈ
ЈЁ5Ј©µ№ЦГВ©¶·µДЧчУГ ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
ОЄСРѕїНУлЕЁБтЛбµД·ґУ¦Ј¬Ді»ЇС§РЛИ¤РЎЧйЅшРРИзПВКµСйЎЈ
КµСйўсЎЎ·ґУ¦ІъОпµД¶ЁРФМЅѕї
КµСйЧ°ЦГИзНјЛщКѕЎЈЈЁ№М¶ЁЧ°ЦГТСВФИҐЈ©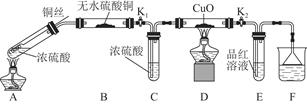
ЈЁ1Ј©AЦР·ґУ¦µД»ЇС§·ЅіМКЅОЄ ЎЈ
ЈЁ2Ј©FЙХ±ЦРµДИЬТєНЁіЈКЗ ЎЈ
ЈЁ3Ј©КµСй№эіМЦРЈ¬ДЬЦ¤ГчЕЁБтЛбЦРБтФЄЛШµДСх»ЇРФЗїУЪЗвФЄЛШµДПЦПуКЗ ЎЈ
ЈЁ4Ј©КµСйЅбКшєуЈ¬Ц¤ГчAЧ°ЦГКФ№ЬЦР·ґУ¦ЛщµГІъОпКЗ·сє¬УРНАлЧУµДІЩЧч·Ѕ·ЁКЗ ЎЈ
ЈЁ5Ј©ОЄЛµГчЕЁБтЛбЦРµДЛ®КЗ·сУ°ПмBЧ°ЦГПЦПуµДЕР¶ПЈ¬»№РлЅшРРТ»ґОКµСйЎЈКµСй·Ѕ°ёОЄ ЎЈ
КµСйўтЎЎ·ґУ¦ІъОпµД¶ЁБїМЅѕї
ЈЁ6Ј©ФЪНУлЕЁБтЛб·ґУ¦µД№эіМЦРЈ¬·ўПЦУРєЪЙ«ОпЦКіцПЦЈ¬ѕІйФДОДПЧ»сµГПВБРЧКБПЎЈ
ЧКБП1Јє
| БтЛб/molЎ¤LЈ1 | єЪЙ«ОпЦКіцПЦµДОВ¶И/Ўж | єЪЙ«ОпЦКПыК§µДОВ¶И/Ўж |
| 15 | Фј150 | Фј236 |
| 16 | Фј140 | Фј250 |
| 18 | Фј120 | І»ПыК§ |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
Ді»ЇС§РЛИ¤РЎЧйОЄСйЦ¤NO2µДСх»ЇРФєНNOµД»№ФРФЈ¬ЙијЖБЛИзПВЧ°ЦГЦЖИЎNO2єНNOЈ¬ІўСйЦ¤ЖдРФЦКЈє
(1)РґіцјЧЦР·ґУ¦µДАлЧУ·ЅіМКЅЈє_________________________________Ј¬
ТТЦРµДПЦПуКЗ_________________________________________________Ј¬
їЙЦ¤ГчNO2µДСх»ЇРФЈ»ФЪ±ыЦР№ДИлїХЖшєуµДПЦПуКЗ_______________Ј¬їЙЦ¤ГчNOµД»№ФРФЎЈ
(2)КµСйЗ°±ыЦРідВъЛ®µДЧчУГКЗ___________________________________
(УГ·ґУ¦µД»ЇС§·ЅіМКЅєНјтТЄОДЧЦ»Шґр)ЎЈ
(3)РЎ»Є¶ФЙПКцКµСйЙијЖМбіцБЛЦКТЙЈ¬ЛыИПОЄТТЦРµДПЦПуІ»ЧгТФЦ¤ГчNO2µДСх»ЇРФЈ¬ЛыµДАнУЙКЗ___________________________________________ЎЈ
ДгИПОЄФхСщІЕДЬЧјИ·Ц¤ГчNO2µДСх»ЇРФЈї____________________________________(јтТЄ»ШґріцФАнєНПЦПујґїЙ)ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
КµСйКТУГЧгБїMnO2УлЕЁСОЛб·ґУ¦ЦЖИЎВИЖшЈ¬ЖдЧ°ЦГИзНј1ЛщКѕЈє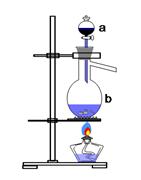

Нј1 Нј2
ЈЁ1Ј©Нј1ЦРТЗЖчaµДГыіЖКЗЈє Ј»ТЗЖчbµДГыіЖКЗЈє Ј»bЦРјУИлЛйґЙЖ¬µДЧчУГКЗЈє ЎЈ
ЈЁ2Ј©ЗлРґіцТЗЖчbЦР·ўЙъµД·ґУ¦µДАлЧУ·ЅіМКЅЈє
ЈЁ3Ј©јмІйЧ°ЦГµДЖшГЬРФЦ®єуµДІЩЧчТАґОКЗЈє Ўў Ўў ЎЈЈЁМоРтєЕЈ©
AЈ®ПтЙХЖїЦРјУИлMnO2·ЫД©
BЈ®јУИИ
CЈ®ПтЙХЖїЦРјУИлЕЁСОЛб
ЈЁ4Ј©ёГ·ґУ¦»бТтОЄСОЛбЕЁ¶ИПВЅµ¶шНЈЦ№ЎЈОЄБЛІв¶Ё·ґУ¦ІРБфТєЦРСОЛбµДЕЁ¶ИЈ¬ДіМЅѕїРЎЧйМбіцПВБРКµСй·Ѕ°ёЈє
ўЩјЧН¬С§µД·Ѕ°ёОЄЈєУлЧгБїAgNO3ИЬТє·ґУ¦Ј¬іЖБїЙъіЙіБµнµДЦКБїЎЈ
ўЪТТН¬С§µД·Ѕ°ёОЄЈєУлЧгБїµДРї·ґУ¦Ј¬ІвБїЙъіЙЖшМеµДМе»эЈ¬КµСйЧ°ЦГИзНј2ЛщКѕЈЁјРіЦЧ°ЦГТСВФИҐЈ©ЎЈК№YРО№ЬЦРµДІРБфИЬТєУлРїБЈ·ґУ¦µДХэИ·ІЩЧчКЗ ЈЁЎ°РїБЈЧЄТЖµЅІРБфИЬТєЦРЎ±»тЎ°ІРБфИЬТєЧЄТЖµЅРїБЈЦРЎ±Ј©ЎЈФЪХэИ·¶БИЎБїЖш№Ь¶БКэК±Ј¬КУПЯТЄЖЅКУЈ¬ТЄЧўТвК№В©¶·ТєГжУлБїЖш№ЬЦРТєГжПаЖЅЈ¬іэґЛНв»№РлЧўТвЈє ЎЈ
БЅЦЦ·Ѕ°ёОТИПОЄ ЈЁМојЧ»тТТЈ©Н¬С§µД·Ѕ°ёїЙРРЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
ДіКµСйРЎЧйЙијЖБЛПВБРЧ°ЦГЅшРР°±µДґЯ»ЇСх»ЇКµСйЎЈ
ЈЁ1Ј©іЈОВПВЈ¬УГ5Ј®8 mol/LµД°±Л®К±КµСйПЦПуГчПФЈ¬ПЦУГЕЁ°±Л®ЕдЦЖёГЕЁ¶ИµД°±Л®480mLЈ¬РиТЄЙХ±ЎўІЈБ§°фЈ¬»№РиТЄµДІЈБ§ТЗЖчУР ЎЈ
ЈЁ2Ј©јЧґ¦Т©Ж·µДГыіЖОЄ_____________ЎЈ
ЈЁ3Ј©КµСйК±Ј¬ПИЅ«ґЯ»ЇјБјУИИЦБємИИЈ¬ФЪІ»¶П№ДИлїХЖшµДЗйїцПВЈ¬ПЁГрѕЖѕ«µЖЈ¬·ґУ¦ИФДЬјМРшЅшРРЈ¬ЛµГч·ґУ¦КЗ_____ЈЁМоЎ°ОьЎ±»тЎ°·ЕЎ±Ј©ИИ·ґУ¦Ј¬»ЇС§·ЅіМКЅОЄ Ј»ТТґ¦јУИлОЮЛ®ВИ»ЇёЖЈ¬ЙХЖїЦР·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅОЄ___ Ј»Ч¶РОЖїЦРІъЙъµДПЦПуОЄ _ЎЈ
ЈЁ4Ј©Из№ыИ±ЙЩТТґ¦µДёЙФп№ЬЈ¬Ѕ«·ґУ¦єуµДЖшМеЦ±ЅУНЁИлЙХЖїЈ¬ФтЙХЖїЦРІъЙъµДПЦПуОЄ ЎЈ
ЈЁ5Ј©ПЦУГГѕУлКЇД«Ччµзј«Ј¬ЕЁВИ»Їп§ИЬТєЧчµзЅвТє№№іЙФµзіШЈ¬Хэј«µДµзј«·ґУ¦КЅОЄ ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
ОЄМЅѕїFe(NO3)2µИПхЛбСОИИ·ЦЅвІъОпєНІъОпµДРФЦКЈ¬Ді»ЇС§РЎЧйїЄХ№ИзПВМЅѕїРФС§П°Јє
ЎѕІйФДЧКБПЎїЅрКф»оЖГРФІ»Н¬Ј¬ЖдПхЛбСО·ЦЅвІъОпІ»Н¬ЎЈ
ЈЁ1Ј©KЎъNa»оЖГЅрКфµДПхЛбСО·ЦЅвЙъіЙСЗПхЛбСОєНСхЖшЈ»
ЈЁ2Ј©MgЎъCuµИЅП»оЖГЅрКфµДПхЛбСО·ЦЅвЙъіЙСх»ЇОпЎўNO2єНO2Ј»
ЈЁ3Ј©HgТФєуІ»»оЖГЅрКфµДПхЛбСО·ЦЅвЙъіЙЅрКфµҐЦКЎўNO2єНO2ЎЈ
2KNO3 2KNO2Ўь+O2Ўь
2KNO2Ўь+O2Ўь
2Cu(NO3)2 2CuO+4NO2Ўь+O2Ўь
2CuO+4NO2Ўь+O2Ўь
2AgNO3 2Ag+2NO2Ўь+O2Ўь
2Ag+2NO2Ўь+O2Ўь
ЎѕКµСйТ»ЎїМЅѕїFe(NO3)2ИИ·ЦЅв№ММеІъОпЦРFeФЄЛШµДјЫМ¬ЎЈёГРЎЧйјЧН¬С§Ѕ«ЖдИЬУЪЧг
БїµДПЎH2SO4µГµЅПаУ¦БЅ·ЭИЬТєЈ¬ЅшРРТФПВМЅѕїКµСйЎЈ
ЎѕМбіцІВПлЎїІВПлТ»ЈєFeФЄЛШЦ»ПФ+2јЫЈ»
ІВПл¶юЈєFeФЄЛШЦ»ПФ+3јЫЈ»
ІВПлИэЈєFeФЄЛШ____ _____ЎЈ
ЎѕКµСйІЩЧчЎїўЩПтТ»·ЭИЬТєЦРµОИлKSCNИЬТєўЪЅ«ПЎЛбРФKMnO4ИЬТєЦРµОИлБнТ»·ЭИЬТє
ЎѕКµСйПЦПуЎїКµСйўЩ Ј»КµСйўЪ ЎЈ
ЎѕКµСйЅбВЫЎїІВПл¶юіЙБўЈ¬ФтFe(NO3)2·ЦЅвµД»ЇС§·ЅіМКЅКЗ ЎЈ
ЎѕКµСй¶юЎїМЅѕїFe(NO3)2ИИ·ЦЅвЖшМеІъОпµДРФЦКЎЈРЎЧйТТЎў±ыН¬С§ЅшРРБЛИзПВНјЛщКѕµДКµСйЈЁКХјЇК±ІЩЧчЗЎµ±Ј¬јёєхГ»УРїХЖшЈ©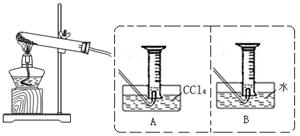
КµСйІЅЦиЈєўЩБ¬ЅУТЗЖчЈ»ўЪјмІйЧ°ЦГЖшГЬРФЈ»ўЫИЎТ»¶ЁЦКБїFe(NO3)2Ч°УЪґуКФ№ЬЈ¬ІўЦШ
РВБ¬ЅУєГТЗЖчЈ»ўЬјУИИЈ»ўЭЎЎ
ЈЁ1Ј©ТТН¬С§К№УГAЧ°ЦГКХјЇЖшМеЈ¬ЗЎєГКХјЇµЅіЈОВіЈС№ПВ27mLµДємЧШЙ«ЖшМеЈ¬ОЄИ·±ЈКэѕЭµДЧјИ·РФЈ¬¶БКэК±±ШРл ЎЈ
ЈЁ2Ј©ТТН¬С§УГґш»рРЗДѕМхјмСйБїНІДЪЖшМеК±Ј¬·ўПЦДѕМхИјЙХЈ¬ЗТСХЙ«±дЗіЙхЦБОЮЙ«Ј¬ПВБРЕР¶ПЦРХэИ·µДКЗ ЎЈ
aЈ®ЖшМеЦРЦ»УРNO2 bЈ®ЖшМеКЗO2ЎўNO2µД»мєПОп
cЈ®Ц§іЦИјЙХµДЖшМеЦ»УРO2 dЈ®NO2Ц§іЦИјЙХ
ЈЁ3Ј©±ыН¬С§ИЎµИЦКБїµДFe(NO3)2К№УГBЧ°ЦГКХјЇЖшМеЈ¬їЙКХјЇµЅ mLЖшМеЎЈ
ЎѕКµСйИэЎїМЅѕї№ММе»мєПОпµДіЙ·ЦЎЈРЎЧй¶ЎН¬С§ИЎKNO3ЎўCu(NO3)2ЎўFe(NO3)2µД»мєП·ЫД©ід·ЦјУИИєуУГЕЕЛ®·ЁОґКХјЇµЅИОєОЖшМеЈ¬ФтKNO3ЎўCu(NO3)2ЎўFe(NO3)2µДОпЦКµДБїЦ®±ИїЙДЬКЗЈЁ Ј©
| AЈ®1:2:2 | BЈ®2:1:3 | CЈ®1:2:3 | DЈ®3:8:6 |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
Ді»ЇС§їОНв»о¶ЇРЎЧйНЁ№эКµСйСРѕїNO2µДРФЦКЈ®
ТСЦЄЈє2NO2+2NaOHЁTNaNO3+NaNO2+H2O
АыУГНј1ЛщКѕЧ°ЦГМЅѕїNO2ДЬ·с±»NH3»№ФЈЁK1ЎўK2ОЄЦ№Л®јРЈ¬јРіЦ№М¶ЁЧ°ЦГВФИҐЈ©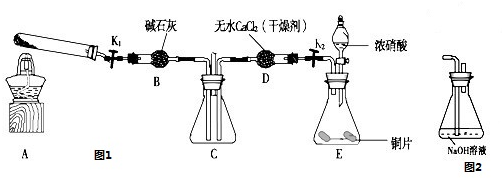
ЈЁ1Ј©EЧ°ЦГЦРЦЖИЎNO2·ґУ¦µДАлЧУ·ЅіМКЅКЗ
ЈЁ2Ј©ёГКµСйЦРЦЖИЎ°±ЖшК±ИфЦ»УГТ»ЦЦКФјБЈ¬ґУПВБРОпЦКЦРСЎИЎ ЈЁ Ј©
aЈ®NH4HCO3 bЈ®NH4Cl cЈ®ЕЁ°±Л®
ЈЁ3Ј©ИфNO2ДЬ№»±»NH3»№ФЈ¬Ф¤ЖЪ№ЫІмµЅCЧ°ЦГЦРµДПЦПуКЗ
ЈЁ4Ј©ґЛКµСйЧ°ЦГґжФЪТ»ёцГчПФµДИ±ПЭКЗ
ЈЁ5Ј©МЅѕїNO2ДЬ·сУлNa2O2·ўЙъСх»Ї»№Ф·ґУ¦Ј®ОЄБЛСйЦ¤NO2ДЬ±»Na2O2Сх»ЇЈ¬ёГРЎЧйͬѧѡУГBЎўDЎўEЧ°ЦГЈ¬Ѕ«BЦРµДТ©Ж·ёь»»ОЄNa2O2Ј¬БнСЎFЧ°ЦГЈЁИзНј2ЛщКѕЈ©Ј¬ЦШРВЧйЧ°Ј¬ЅшРРКµСйЈ®Ч°ЦГµДєПАнБ¬ЅУЛіРтКЗ
ЈЁ6Ј©КµСй№эіМЦРЈ¬BЧ°ЦГЦРµ»ЖЙ«·ЫД©Цр ЅҐ±діЙ°ЧЙ«Ј®ѕјмСйЈ¬ёГ°ЧЙ«ОпЦКОЄґїѕ»ОпЈ¬ЗТОЮЖдЛыОпЦКЙъіЙЈ®НЖІвBЧ°ЦГЦР·ґУ¦µД»ЇС§·ЅіМКЅОЄ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
ПВНјОЄКµСйКТЦЖИЎґїѕ»ЎўёЙФпµДCl2Ј¬ІўЅшРРјмСйCl2РФЦККµСйµДЧ°ЦГЎЈЖдЦРEЖїЦР·Е
УРёЙФпємЙ«ІјМхЈ»FЦРОЄННшЈ¬УТ¶ЛОЄТ»НЕГЮ»ЁЎЈ
|
|
 КФ»ШґрЈє
КФ»ШґрЈєІйїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com