
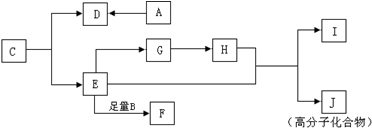
 ��JΪ
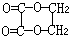
��JΪ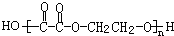 ��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬�ݴ˽��
��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬�ݴ˽�� ��JΪ
��JΪ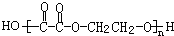 ��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬
��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬ ���ʴ�Ϊ����ȩ��
���ʴ�Ϊ����ȩ�� ��
��| Ũ���� |
| �� |
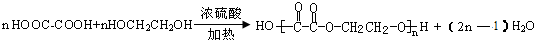 ���������۷�Ӧ��
���������۷�Ӧ��| Ũ���� |
| �� |
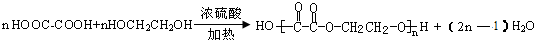 �����۷�Ӧ��
�����۷�Ӧ��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��pH=9�İ�ˮ��Һϡ��1000�� |
| B��pH=9���ռ���Һϡ��1000�� |
| C��pH=5��������Һϡ��1000�� |
| D��pH=5���Ȼ����Һϡ��1000�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ħ������ M g/mol |
| B������Ħ����� Vm L/mol |
| C���ܽ�� S g/100g |
| D���ܶ� �� g/cm3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������������������Һ |
| B��ϡ����������������Һ |
| C������������Һ������������Һ |
| D�������백ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ�� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ�� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� �� | �������Լ� | �й����ӷ���ʽ |
| HNO3��H2SO4�� | ||
| Cu��Fe�� | ||
| ZnSO4��CuSO4�� | ||
| NaCl��Na2CO3�� | ||
| FeCl2��FeCl3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ͨ��CH4�ĵ缫Ϊ���� |
| B���õ��ʹ��һ��ʱ���Ӧ����KOH |
| C����������������Ӧ |
| D��ȼ�ϵ�ع���ʱ����Һ�е�OH-�������ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com