������8.34g FeS0
4?7H
20��Ʒ���ʵ���=
=0.03mol������m��H
20��=0.03mol��7��18g/mol=3.78g���羧��ȫ��ʧȥ�ᾧˮ�����������ӦΪ8.34g-3.78g=4.56g����֪�ڼ��ȵ�373��֮ǰ������ʧȥ���ֽᾧˮ����������ı仯��ȷ���ڲ�ͬ�¶�ʱ���Ⱥ����Ļ�ѧʽ��������633��ʱ�����������Ϊ2.40g������n��Fe��=n�� FeS0
4?7H
20��=0.03mol��m��Fe��=0.03mol��56g/mol=1.68g���������m��O��=2.40g-1.68g=0.72g��n��O��=
=0.045mol����n��Fe����n��O��=0.03mol��0.045mol=2��3�����������Q�Ļ�ѧʽΪFe
2O
3���Դ˽����⣮
���
�⣺8.34g FeS0
4?7H
20��Ʒ���ʵ���=
=0.03mol������m��H
20��=0.03mol��7��18g/mol=3.78g���羧��ȫ��ʧȥ�ᾧˮ�����������ӦΪ8.34g-3.78g=4.56g����֪�ڼ��ȵ�373��֮ǰ������ʧȥ���ֽᾧˮ��
A���¶�Ϊ78��ʱ����������Ϊ6.72g������m��FeS0
4��=0.03mol��152g/mol=4.56g��m��H
20��=6.72g-4.56g=2.16g��n��H
20��=
=0.12mol����n��H
20����n��FeS0
4��=0.12mol��0.03mol=4��1����ѧʽΪFeSO
4?4H
2O����A��ȷ��
B���¶�Ϊl59��ʱ����������Ϊ5.10g������m��FeS0
4��=0.03mol��152g/mol=4.56g��m��H
20��=5.10g-4.56g=0.54g��n��H
20��=
=0.03mol����n��H
20����n��FeS0
4��=0.03mol��0.03mol=1��1����ѧʽΪFeSO
4?H
2O����B��ȷ��
C��m��FeS0
4��=0.03mol��152g/mol=4.56g�����ڸ���������������N�õ�P�Ļ�ѧ����ʽΪFeSO
4?H
2O
FeS0
4+H
20����C����
D������P��Ӧ�¶�Ϊ373�����Ϊ4.56g����������������650�棬������633��ʱ�����������Ϊ2.40g������n��Fe��=n�� FeS0
4?7H
20��=0.03mol��m��Fe��=0.03mol��56g/mol=1.68g���������m��O��=2.40g-1.68g=0.72g��n��O��=
=0.045mol����n��Fe����n��O��=0.03mol��0.045mol=2��3�����������Q�Ļ�ѧʽΪFe
2O
3�����Ļ��ϼ����ߣ�������Ļ��ϼ۽��ͣ���SO
2��SO
3�����ʵ����ֱ�Ϊx��y����
| | x+y=0.03 | | 64x+80y=4.56-2.40 |
| |
�����
��������P��Q����ʽΪ��2FeSO
4 Fe
2O
3+SO
2��+SO
3������������ɫ�������ɣ���D����
��ѡ��CD��

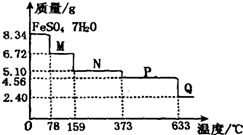 ȡ8.34g FeS04?7H20��Ʒ���ȣ���������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ�������������в���ȷ���ǣ�������
ȡ8.34g FeS04?7H20��Ʒ���ȣ���������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ�������������в���ȷ���ǣ�������


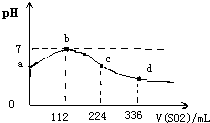 ��״���£���100mlH2S������Һ��ͨ��SO2���壬������ҺpH�仯��ͼ��������ʾ�����з�����ȷ���ǣ�������
��״���£���100mlH2S������Һ��ͨ��SO2���壬������ҺpH�仯��ͼ��������ʾ�����з�����ȷ���ǣ�������