
����Ŀ���о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
��1����֪��1 mol H2��1 mol O2��Һ̬ˮ��1 mol O��H��ʹ֮��Ϊ��̬ԭ������������ֱ�Ϊ436 kJ��496 kJ��462 kJ��CH3OH(g)��ȼ����Ϊ627 kJ��mol��1����CO2(g)��3H2(g)��CH3OH(g)��H2O(l) H��___________kJ��mol��1
��2����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g)�� 6H2(g) ![]() CH3OCH3(g)��3H2O(l)
CH3OCH3(g)��3H2O(l)
�� �÷�Ӧƽ�ⳣ������ʽK��_______________��
�� ��֪��ijѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת������ͼ��ʾ���÷�Ӧ��H________0 (����������������)��
���¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]����K��_____(����������������С������������)��
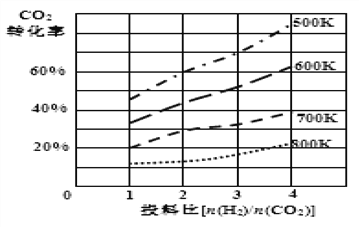
��ij�¶��£������һ�����ܱ�������ͨ��CO2(g)��H2(g)����������Ӧ�����������������ٷ����仯ʱ����֤���������淴Ӧ�ﵽƽ�������__________��
A��������̼��Ũ�� B�������е�ѹǿ
C��������ܶ� D��CH3OCH3��H2O�����ʵ���֮��
��3���Լ��ѡ�����������������ҺΪԭ�ϵ�ȼ�ϵ��Ϊ��Դ����ʯīΪ�缫���500mL���з�̪��NaCl��Һ��װ����ͼ��ʾ��
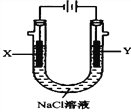
��д����������Y�缫�����۲쵽������______________����ȼ�ϵ������2.8L O2(��״����)ʱ�������ʱNaCl��Һ��pH=________(������Һ��������䣬����ȫ������Һ���ݳ�)��
���𰸡� ��93 c(CH3OCH3)/ [c2(CO2)��c6(H2)] �� ���� ABC Y�缫������Һ��������������ϲ��ֳʻ���ɫ 14
��������(1)��֪��1 mol H2��1 mol O2��Һ̬ˮ��1 mol O��H��ʹ֮��Ϊ��̬ԭ������������ֱ�Ϊ436 kJ��496 kJ��462 kJ�����˿�д��H2ȼ������Һ̬ˮ���Ȼ�ѧ����ʽΪ��2H2(g)��O2(g)= 2H2O(l) ��H=��480kJ��mol��1����CH3OH(g)��ȼ����Ϊ627 kJ��mol��1���ɵ�CH3OH(g)ȼ�յ��Ȼ�ѧ����ʽΪ��CH3OH(g)��3/2O2(g)=CO2(g)��2H2O(l) ��H=��627kJ��mol��1���ɸ�˹���ɿɽ��Т١�3/2���ڣ�����CO2(g)��3H2(g)��CH3OH(g)��H2O(l) H����93 kJ��mol��1��
(2)�ٸ���ƽ�ⳣ���ı���ʽ����ɵ�����Ӧ2CO2(g)�� 6H2(g) ![]() CH3OCH3(g)��3H2O(l)��ƽ�ⳣ������ʽK��c(CH3OCH3)/ [c2(CO2)��c6(H2)]���ڸ���ͼ��������¶�Խ�ߣ�CO2��ת����Խ�ͣ�˵�������¶ȣ�ƽ�������ƶ�����÷�ӦΪ���ȷ�Ӧ����H�� 0�����¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]��CO2��ת���ʼ�С��˵��ƽ�������ƶ�����ƽ�ⳣ��K�Dz���ģ���Ϊƽ�ⳣ��Kֻ���¶ȵĸı���仯���۸��ݻ�ѧƽ��״̬���������������£�A����c(CO2)����ʱ��˵����Ӧ�Ѵ�ƽ�⣬��A��ȷ��B�������һ�����ܱ������У��÷�Ӧǰ�������������ȣ����Ե�ѹǿ����ʱ��˵����Ӧ�Ѵ�ƽ�⣬��B��ȷ��C�����ݦ�=m/V�������һ�����ܱ������У�V���䣬��������ˮ��Һ�壬���������������m��仯�������ܶȦѵĸı䣬�ʵ��ܶȦѲ���ʱ��˵����Ӧ�Ѵ�ƽ�⣬��C��ȷ��D�����Ǵӷ�Ӧ��CO2(g)��H2(g)��ʼ���еķ�Ӧ������������CH3OCH3��H2O�����ʵ���֮��ʼ�ն���1:3���Dz���ģ���CH3OCH3��H2O�����ʵ���֮�Ȳ���ʱ������˵����Ӧ�Ѵ�ƽ�⣬��D������ȷ��ΪABC��
CH3OCH3(g)��3H2O(l)��ƽ�ⳣ������ʽK��c(CH3OCH3)/ [c2(CO2)��c6(H2)]���ڸ���ͼ��������¶�Խ�ߣ�CO2��ת����Խ�ͣ�˵�������¶ȣ�ƽ�������ƶ�����÷�ӦΪ���ȷ�Ӧ����H�� 0�����¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]��CO2��ת���ʼ�С��˵��ƽ�������ƶ�����ƽ�ⳣ��K�Dz���ģ���Ϊƽ�ⳣ��Kֻ���¶ȵĸı���仯���۸��ݻ�ѧƽ��״̬���������������£�A����c(CO2)����ʱ��˵����Ӧ�Ѵ�ƽ�⣬��A��ȷ��B�������һ�����ܱ������У��÷�Ӧǰ�������������ȣ����Ե�ѹǿ����ʱ��˵����Ӧ�Ѵ�ƽ�⣬��B��ȷ��C�����ݦ�=m/V�������һ�����ܱ������У�V���䣬��������ˮ��Һ�壬���������������m��仯�������ܶȦѵĸı䣬�ʵ��ܶȦѲ���ʱ��˵����Ӧ�Ѵ�ƽ�⣬��C��ȷ��D�����Ǵӷ�Ӧ��CO2(g)��H2(g)��ʼ���еķ�Ӧ������������CH3OCH3��H2O�����ʵ���֮��ʼ�ն���1:3���Dz���ģ���CH3OCH3��H2O�����ʵ���֮�Ȳ���ʱ������˵����Ӧ�Ѵ�ƽ�⣬��D������ȷ��ΪABC��
(3)��װ��ͼ��֪��Y�缫���Դ������������Ϊ���������NaCl��Һ��������ӦʽΪ2Cl����2e��=Cl2�������Կɹ۲쵽������Ϊ�缫�������ݲ������ϲ�����ʻ���ɫ����ȼ�ϵ������2.8L O2(��״����)ʱ����·��ת�Ƶĵ���n(e- )=![]() =0.5mol,���ݵ���ת���غ㣬��ϵ��NaCl��Һ�ķ�Ӧ����ʽ2NaCl��2H2O
=0.5mol,���ݵ���ת���غ㣬��ϵ��NaCl��Һ�ķ�Ӧ����ʽ2NaCl��2H2O![]() 2NaOH��H2����Cl2��,�ɵô�ʱ��Һ������n(OH-)=0.5mol��c(OH-)=0.5mol/0.5L=1.0mol/L��������Һ��pH=14��
2NaOH��H2����Cl2��,�ɵô�ʱ��Һ������n(OH-)=0.5mol��c(OH-)=0.5mol/0.5L=1.0mol/L��������Һ��pH=14��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʣ�����������ʡ������˳�����е��ǣ� ��
A.�ռҺ̬�������
B.��ʯ�ҡ����ס���ʯ��
C.�ɱ��������Ȼ���
D.����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________��
��2���ձ���������ֽ����������________________��
��3�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������________________(���ȡ�����ȡ�)�������к���___________(���ȡ�����ȡ�)��
��4������ͬŨ�Ⱥ�����İ�ˮ��NH3��H2O������NaOH��Һ��������ʵ�飬 ��õ��к��ȵ���ֵ��________________�����ƫ����ƫС��������Ӱ�족����
������1����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��__________________________��

��2�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣��֪��C(s��ʯī)+O2(g)=CO2(g) ��H1=-393.5kJ��mol-1
2H2(g)+O2(g)=2H2O(l) ��H2=-571.6kJ��mol-1
2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l) ��H3=-2599kJ��mol-1
���ݸ�˹����������298Kʱ��C(s��ʯī)��H2(g)����1mol C2H2(g)��Ӧ���ʱ�����H=___________��
��3��1.3gC2H2��ȫȼ������Һ̬ˮ��CO2���ų�62kJ������д��C2H2ȼ�յ��Ȼ�ѧ����ʽ��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������⣬�����¡��״�������ú����Һ������壬������ȼ�ϵ�ص�ȼ��������֪��ȼ��ʱ�������з�Ӧ��N2H4+O2![]() N2+2H2O����PtΪ�缫��������Ϊ�������Һ�����ȼ�ϵ�أ����й�����ȼ�ϵ�ص�˵���в���ȷ�� )
N2+2H2O����PtΪ�缫��������Ϊ�������Һ�����ȼ�ϵ�أ����й�����ȼ�ϵ�ص�˵���в���ȷ�� )
A. ����ȼ�ϵ�صĸ�����Ӧ�O2��������Ӧ��
B. ��ȼ�ϵ�ص�������ӦΪ O2 + 2H2O + 4e��= 4OH��
C. ��ȼ�ϵ�صĸ�����ӦΪ��N2H4 ��4e��=N2��+4H+
D. ��ع���������H+�������ƶ�����H+���ʵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ȣ�SO2Cl2����һ����Ҫ�Ļ����Լ���ʵ���Һϳ������ȵ�ʵ��װ������ͼ��ʾ��

��֪���� SO2(g) + Cl2(g)![]() SO2Cl2(l) ��H = ��97.3 kJ/mol��
SO2Cl2(l) ��H = ��97.3 kJ/mol��
�� ������ͨ��������Ϊ��ɫҺ�壬�۵�Ϊ��54.1�棬�е�Ϊ69.1�棬�ڳ�ʪ�����С����̡���100�����Ͽ�ʼ�ֽ⣬���ɶ�����������������ڷ���Ҳ�ᷢ���ֽ⡣
�ش��������⣺
��1��װ�ü�Ϊ����װ�ã������ṩ������������A��ʢ�ŵ��Լ�Ϊ______________��װ�ñ��л���̿��������______________��
��2������ͼ�����ڻ�����ȱװ�ã���ע������Լ���������
��3���Ȼ��ᣨClSO3H�����ȷֽ⣬Ҳ���Ƶ�������������һ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________���������ķ����ǣ�����ĸ��_________��
A���ؽᾧ B������ C������ D����ȡ
��4��Ϊ��߱�ʵ���������ȵIJ��ʣ���ʵ���������Ҫע���������________������ţ���
�� ��ͨ����ˮ����ͨ�� �� �����������ʣ��������˿�
�� ��������ƿ���̣����ʵ����� �� ����������ƿ
��5��������̿���ڵ�������SO2��Cl2Ҳ�ɷ�����Ӧ���ֽ�SO2��Cl2����һ������ͨ��ˮ�У������ һ����ʵ����֤�����Ƿ�ǡ����ȫ��Ӧ�� ����Ҫ����ʵ�鲽�衢����ͽ��ۣ���
������ѡ����ѡ���Լ����μӷ�̪������������Һ���⻯����Һ��������Һ��Ʒ����Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���밴Ҫ��ش��������⣺
��1���������ӷ���ʽ��ʾ������Һ�ʼ��Ե�ԭ��_____________________________��
��2���������ӷ���ʽ��ʾ������ˮ��ԭ����_____________________________��
��3�����û�ѧ����ʽ��ʾ�ȼҵ�ķ�Ӧԭ����_____________________________��
��4�����û�ѧ����ʽ��ʾ���ȷ������ķ�Ӧԭ����_____________________________��
��5����AlCl3��Һ���ɲ����գ����õ�����Ҫ���������_______________��
��6����25 ���£���a mol��L��1�İ�ˮ��0.01 mol��L��1������������ϣ���Ӧƽ��ʱ��Һ��c(NH4+ )��c(Cl��)������Һ��_________(������������������������)�ԣ��ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA ��ʾ�����ӵ�������ֵ������˵����ȷ����
A. �ڷ�ˮ�е��뺬0.1molFeC13�ı�����Һ���Ƶý�����Ϊ0.1 NA
B. ��0.4mol HNO3��ϡ����������Fe��Ӧ��ת�Ƶ�����Ϊ1.2 NA
C. 120 gNaHSO4��MgSO4�ľ�����������������ΪNA
D. ��״���£�44.8LSO2������O2��Ӧ���ɵ�SO3������Ϊ2 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����Ҫ����100 mL 2 mol/L NaCl��Һ����ش��������⣺
��1�����ƹ�������Ҫʹ�õ���Ҫ�������������ձ�������������ͷ�ιܡ���Ͳ��________��
��2����������ƽ��ȡ�Ȼ��ƹ��壬������Ϊ__________ g��
��3��������Ҫ�����������ȷ˳����___________������ţ���
�ٳ�ȡһ���������Ȼ��ƣ������ձ��У�����������ˮ�ܽ�
�ڼ�ˮ��Һ��������ƿ���̶�����1~2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶�������
�۽���Һת�Ƶ�����ƿ��
�ܸǺ�ƿ�����������µߵ���ҡ��
������������ˮϴ���ձ��ڱںͲ�����2~3�Σ�ϴ��Һת�Ƶ�����ƿ��
��4�����ʵ�������ȱ�ٲ���ݣ������������Һ�����ʵ���Ũ��_______���ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ����������ʱ��������ƿ�̶��ߣ������������Һ�����ʵ���Ũ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��DΪ������Ԫ�ع��ɵ��������ʣ�����������ת����ϵ����DΪǿ����ʣ�����������ʿ���ʡ�ԣ�����˵������ȷ���ǣ� ��
![]()
A. ��AΪ�ǽ������ʣ���Dһ��Ϊ���������
B. ��AΪ�������ʣ������A��Ԫ��һ��λ�ڵ������ڵ�IA��
C. ����AΪ���ʻ��ǻ����D���п�����ͬһ�����ʣ������ʵ�Ũ��Һ�ڳ�������ʹ�����������ۻ�
D. ��A�ǹ��ۻ����A��ˮ��Һһ���Լ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com