
����Ŀ������һ�ְ뵼����ϣ�����ϡɢ����������Ϊ���ִ���ҵ���������˼�����ά���أ������˼��漣�����������ǵ��������²��ϵ�֧�Ų��ϡ����ڻ���ͭ������Ҫ����ΪCu2Te��Cu��CuSO45H2O��Au��Ag�ȣ�Ϊԭ����ȡ���Ʊ�TeO2�͵���Te�Ĺ���������ͼ��ʾ��
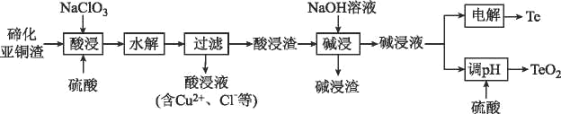
��֪����ˮ������ӦΪH2TeO3(������)=TeO2��+H2O��
�ش��������⣺
��1��Cu2Te��Te�Ļ��ϼ�Ϊ___��
��2���������ʱ��Ҫʹ6molCu�ܽ⣬��Cu��Ӧ��NaC1O3�����ʵ���Ϊ___��
��3��д���������ʱCu2Te����ת�������ӷ���ʽ��___��
��4��ȡ�ڻ���ͭ��100g����������������������Ũ�ȶ��ڻ���ͭ������Ч����Ӱ����ͼ��ʾ��

ѡ����ѵ���������������Ϊ___g��ѡ�������Ũ��ԼΪ___mol/L(����С�����һλ����
��5������������к��еĽ���������Ҫ��___(�ѧʽ�������кܸߵľ������ü�ֵ��
��6�������Һ���������������ҺpH��5.5��������TeO2���ù��̵����ӷ���ʽΪ____��
��7����������ǹ�ҵ���Ʊ���Te�ij��÷������Բ���ְ����ͨ������������������һ���ĵ����ܶȡ��¶��µ����Һ����Ԫ���Խ���Te��ʽ�������������������ĵ缫��ӦʽΪ___��
���𰸡�-2 2mol Cu2Te+4C1O3-+12H+=6Cu2++3H2TeO3+4Cl-+3H2O 50 0.7 Au��Ag TeO32-+2H+= TeO2��+ H2O TeO32-+ 3H2O+ 4e-=Te+6OH-
��������
�ڻ���ͭ������Ҫ����ΪCu2Te��Cu��CuSO45H2O��Au��Ag�ȣ��������������ܽ⣬����Cu��Au��Ag�������ᷴӦ���γɺ���Cu+��Cu2+��Te2+��SO42-����Һ���ټ��������ƣ���������������������Ӿ���ǿ�����ԣ�����Һ��Cu��Cu+����ΪCu2+��Te2+������ת��ΪH2TeO3������ˮ��H2TeO3ת��ΪTeO2�����˺������Һ�к���Cu2+��Cl-���������ijɷ�ΪAu��Ag��TeO2�����������м����������ƣ���TeO2�ܽ�ת��Na2TeO3���ڹ��ˣ��õ�������ͼ��Һ�����������Ҫ����Au��Ag�����Һ��ҪΪNa2TeO3���Լ��Һ���ɵõ�Te������Һ�м����������pHֵ����TeO2���ݴ˷������
(1)�ڻ���ͭCu2Te��Cu Ϊ+1�ۣ�Te���ϼ�Ϊ-2�ۣ�
(2) 6molCuת��ΪCu2+ʧȥ12mole-��1mol NaC1O3ת��ΪCl-�õ�6mol e-����Ҫʹ6molCu�ܽ⣬��Cu��Ӧ��NaC1O3�����ʵ���Ϊ2mol��
(3)д���������ʱ����������������������Ӿ���ǿ�����ԣ� Te2+������ת��ΪH2TeO3��Cu2Te����ת�������ӷ���ʽ��Cu2Te+4C1O3-+12H+=6Cu2++3H2TeO3+4Cl-+3H2O��
(4)ͭ���ڵĽ��������������������������Ӷ�����m(NaC1O3): m(�ڻ���ͭ��)��0.5ʱ��ͭ�����ʱ仯�����ڵĽ����ʴ�������ӣ���ˣ�Ϊ�����ڵ���ʧ��ͭ����Ч���룬ѡ��m(NaC1O3): m(�ڻ���ͭ��)=0.5���ڻ���ͭ��Ϊ100g����NaC1O3��������Ϊ50g��ͭ���ڵĽ�������������Ũ�ȵ���������ߣ�������Ũ������70g/Lʱ��������������Ũ�ȶ�ͭ�Ľ�����Ӱ�첻���������ٽ��ڵ��ܽ⣬��ˣ�ѡ����������Ũ��Ϊ70g/L�����ʵ���Ũ��ԼΪ![]() ��0.7mol/L��
��0.7mol/L��
(5)���ݷ�����Au��Ag�����ȶ����������ᡢ����������Һ��Ӧ���� ����������к��еĽ���������Ҫ��Au��Ag��
(6)���ݷ��������Һ��ҪΪNa2TeO3ͨ�����������ҺpH�����е����ӷ�Ӧ����ʽΪTeO32-+2H+= TeO2��+ H2O��
(7)�����Һ����Ԫ���Խ���Te��ʽ�������������������ĵ缫��ӦʽΪTeO32-+3H2O+ 4e-=Te+6OH-��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D��E����ԭ���������������Ԫ��(ԭ��������С��30)��A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ�Eԭ���������1�������ӣ���������3���ܼ��Ҿ��������ӣ�D��Eͬ���ڣ��۵�����Ϊ2����
(1)D��Ԫ�ط���Ϊ______��A�ĵ��ʷ����������ĸ���Ϊ______��
(2)BԪ�ص��⻯��ķе���ͬ��Ԫ���⻯������ߵģ�ԭ����__________________________________��
(3)A��B��C 3��Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
(4)д����̬Eԭ�ӵļ۵����Ų�ʽ��__________________��

(5)A������⻯����ӵĿռ乹��Ϊ________������Aԭ�ӵ��ӻ�������________��
(6)C��D�γɵĻ�����ľ����ṹ��ͼ��ʾ����֪������ܶ�Ϊ�� g��cm��3�������ӵ�����ΪNA�����߳�a��________cm��(������NA�ļ���ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ����Ҫ�Ļ����м��壬������ҵ�о����ȵ㡣һ���Ը����̿�(��Ҫ�ɷ�Ϊ���̻����PFeS)Ϊԭ���Ʊ������̵Ĺ����������£�

��֪���١���ϱ��ա���������MnSO4��Fe2O3������FeO��Al2O3��MgO��
�ڽ���������ˮ��Һ�е�ƽ��Ũ����pH�Ĺ�ϵ��ͼ��ʾ(25��)��

�۴�ʵ��������Mn2+��ʼ������pHΪ7.54������Ũ�ȡ�10��5mol��L��1ʱ�����ӳ�����ȫ��
��ش�
(1)��ͳ���մ��������̿�ʱ����������ϱ��ա�������ֱ����H2SO4��������ȱ��Ϊ___________��
(2)��������ʱ��������Ӧ�����ӷ���ʽΪ_________________________________����ʡ�ԡ����������裬��ɵĺ����_________________________________��
(3)���кͳ��ӡ�ʱ�����ɳ�������Ҫ�ɷ�Ϊ______________________(�ѧʽ)��
(4)���������ӡ�ʱ����ʹ��Һ�е�Mg2+��Ca2+������ȫ����ά��c(F��)������___________��(��֪��Ksp(MgF2)=6.4��10��10��Ksp(CaF2)=3.6��10��12)
(5)��̼���ᾧ��ʱ��������Ӧ�����ӷ���ʽΪ______________________��
(6)��ϵ�в�����ָ___________�����ˡ�ϴ�ӡ�����
(7)�ö��Ե缫���MnSO4��Һ���Ʊ�MnO2���������������ĵ缫��ӦʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л���F( )�����������Լ�����������������Ҫ��Ӧ�ã���ҵ������ϩ�ͷ����廯����BΪ����ԭ���Ʊ�F��·��ͼ���£�
)�����������Լ�����������������Ҫ��Ӧ�ã���ҵ������ϩ�ͷ����廯����BΪ����ԭ���Ʊ�F��·��ͼ���£�

(1)��ϩ����A��ԭ��������Ϊ100%����X��___________(�ѧʽ)��F�к��������ŵ�����Ϊ___________��
(2)E��F�ķ�Ӧ����Ϊ___________��B�Ľṹ��ʽΪ___________����E������Ϊ�����ᣬ��F��������___________��
(3)д��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_________________________________��
(4)E�ж���ͬ���칹�壬��������������ͬ���칹����___________�֣����к˴Ź���������6��������ʵĽṹ��ʽΪ___________��
���ܷ���ˮ�ⷴӦ��������Ӧ��1mol���������ɻ�ԭ��4 mol Ag
����FeCl3��Һ������ɫ��Ӧ
�۷�����û�м����ұ�������2��ȡ����
(5)����ϩΪ����ԭ�ϣ���ƺϳ�·�ߺϳ�2-��ϩ�ᣬд���ϳ�·�ߣ�______________________(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�����������ó��Ľ�����ȷ����( )
ѡ�� | ʵ����� | ʵ������ | ���� |
A | ������ | ������ʹʪ�����ɫʯ����ֽ��� | �ȴ�����ˮ�е���� |
B | ���������ᴦ���� | �����ɳ�ɫ��Ϊ��ɫ | �Ҵ����������� |
C | �� | ������ɫ���� | ������������� |
D | ����������ȼ�ճ��е�ȼ��Ѹ�����뼯�� | �����������̣�ƿ���к�ɫ�������� |
|
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����գ�����Ȼ��ͨѶ���������ҹ�������ѧκ����Ŷӿ�����һ�ֹ����������������μ�������ֱ���ڶ������������������������������Ĥ���ش��������⣺
(1)����Nԭ�ӵĵ����Ų�ͼ��ʾ��״̬�У������ɵ͵��ߵ�˳����______(����ĸ��ʾ)��
A.![]() B.
B.![]()
C.![]() D.
D.![]()
(2)����Ԫ�ش���ͬ���������ڵ�����Ԫ�غ���Ԫ�صĵ�һ�������ɴ�С��˳����_______(��Ԫ�ط��ű�ʾ)��
(3)![]() �����幹��Ϊ______________������ԭ��Si�Ĺ���ӻ�����Ϊ_____________��
�����幹��Ϊ______________������ԭ��Si�Ĺ���ӻ�����Ϊ_____________��
(4)����Ԫ��״����![]() ��Ϊ�ȵ�������л�����Ϊ____________(��ṹ��ʽ)��
��Ϊ�ȵ�������л�����Ϊ____________(��ṹ��ʽ)��
(5)��Ȼ���к���Ԫ�ص�������һ����Ȼ��أ��仯ѧʽд��![]() ��ʵ�������Ľṹ��Ԫ��������
��ʵ�������Ľṹ��Ԫ��������![]() ������
������![]() ���϶��ɵ�˫��Ԫ����Ӧ��д��
���϶��ɵ�˫��Ԫ����Ӧ��д��![]() ���������ӵĽṹ��ͼ��ʾ�����������ӿ��γ���״�ṹ��
���������ӵĽṹ��ͼ��ʾ�����������ӿ��γ���״�ṹ��
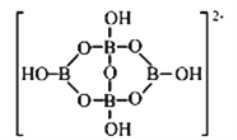
�ٸþ����в����ڵ���������_____________(��ѡ����ĸ)��
A.���Ӽ� B.���ۼ� C.������ D.���»��� E.���
��������ͨ��____________(��������������)�����γ���״�ṹ��
(6)![]() Ӳ�ȴ��۵�ߣ������ṹ�մɲ��ϡ�
Ӳ�ȴ��۵�ߣ������ṹ�մɲ��ϡ�![]() ��
��![]() �ṹ���ƣ���Ƚ϶����۵�ߵͣ���˵�����ɣ�_____________________��
�ṹ���ƣ���Ƚ϶����۵�ߵͣ���˵�����ɣ�_____________________��
(7)��������������ԭ�Ӿ��壬�侧���ṹ��ͼ��ʾ��������ԭ�ӵ���λ��Ϊ____________����֪������������ܶ�Ϊ![]() ��Bԭ�Ӱ뾶Ϊ
��Bԭ�Ӱ뾶Ϊ![]() ��Nԭ�Ӱ뾶Ϊ
��Nԭ�Ӱ뾶Ϊ![]() �������ӵ�������ֵΪ
�������ӵ�������ֵΪ![]() ����þ�����ԭ�ӵĿռ�������Ϊ_____________________(�ú�
����þ�����ԭ�ӵĿռ�������Ϊ_____________________(�ú�![]() �Ĵ���ʽ��ʾ)��
�Ĵ���ʽ��ʾ)��
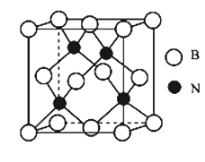
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ۺ���H��һ�־�������ά����ṹ��ʽΪ![]() ���þۺ���ɹ㷺���ڸ���ɲ��Ƭ����ϳ�·������ͼ��ʾ��
���þۺ���ɹ㷺���ڸ���ɲ��Ƭ����ϳ�·������ͼ��ʾ��

��֪����C��D��G��Ϊ�����廯��������о�ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ�
��Diels��Alder��Ӧ��![]() ��
��
��1������A�ķ�Ӧ������________��D��������________��F�����������ŵ�������_________��
��2��B�Ľṹ��ʽ��________����B��C���ķ�Ӧ�У���C�⣬�����ɵ�һ����������______���ѧʽ����
��3��D��G��H�Ļ�ѧ����ʽ��_________��
��4��Q��D��ͬϵ�����Է���������D��14����Q���ܵĽṹ��______�֡����У��˴Ź���������4��壬�ҷ������Ϊ1��2��2��3�Ľṹ��ʽΪ_______����дһ�֣���
��5����֪����Ȳ��1��3������ϩҲ�ܷ���Diels��Alder��Ӧ������1��3������ϩ����ȲΪԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�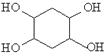 ��д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ������_______��
��д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴ӦA(g)��B(g)![]() 2C(g)�ڲ�ͬ�¶��¾���һ��ʱ�䣬�������C������������¶ȵĹ�ϵ��ͼ��ʾ��
2C(g)�ڲ�ͬ�¶��¾���һ��ʱ�䣬�������C������������¶ȵĹ�ϵ��ͼ��ʾ��

��1����T1��T2�仯ʱ������Ӧ����_______�淴Ӧ����(����>����<������=��)��
��2����T3��T4�仯ʱ������Ӧ����_______�淴Ӧ����(����>����<������=��)��
��3����Ӧ��_________�¶��´ﵽƽ�⡣
��4���˷�Ӧ������ӦΪ_______�ȷ�Ӧ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com