
����Ŀ���������������
(1)д���ɼױ��Ʊ��������ױ��Ļ�ѧ����ʽ��________��
(2)ij�л������ʽ��C3H2O2������ˮ��Һ����������ʹ��ˮ��ɫ�����л���Ľṹ��ʽΪ________��
(3)![]() ���ӽṹ�������________��ԭ�ӹ��档
���ӽṹ�������________��ԭ�ӹ��档
(4)�����м��ַ�Ӧ���ͣ�����ȥ��ȡ���������ܼӳɢݻ�ԭ��ˮ�⣬�ñ�ȩ��ȡ1��2-����������ȷ�ĺϳ�·�����η����ķ�Ӧ�������Ͳ�������________��
A.�ݢ٢ܢ�B.�ܢ٢ܢ�C.�ݢ٢ܢ�D.�ݢۢޢ�
(5)������A����ʽΪC5H8�����Ǻϳ���Ȼ�ĵ��壬����A�ϳɵ���Ȼ��˳ʽ�ṹ��ʽΪ________��
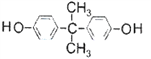
(6)��̼���� ���������ã����������������ɻ��ĵ��粣�����Լ��۾���Ƭ�����̡���Ƭ�ȡ��ϳɾ�̼����������ɫ��ѧԭ��̼�������(
���������ã����������������ɻ��ĵ��粣�����Լ��۾���Ƭ�����̡���Ƭ�ȡ��ϳɾ�̼����������ɫ��ѧԭ��̼�������( )��________(д�����л���Ľṹ��ʽ�����Ͼۺ϶��ɡ�
)��________(д�����л���Ľṹ��ʽ�����Ͼۺ϶��ɡ�
���𰸡� 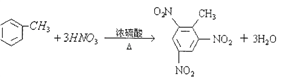 CH2=CH-COOH 20 D
CH2=CH-COOH 20 D 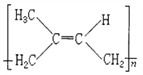
��������(1)�ױ���Ũ���������Ũ���ᷢ��ȡ�����������������ױ����䷴Ӧ�Ļ�ѧ����ʽΪ��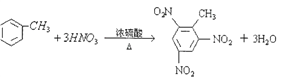 ��(2)����ʽΪC3H4O2���л����ˮ��Һ����������ʹ��ˮ��ɫ����Ϊ��ϩ�ᣬ��ṹ��ʽΪCH2=CH-COOH��
��(2)����ʽΪC3H4O2���л����ˮ��Һ����������ʹ��ˮ��ɫ����Ϊ��ϩ�ᣬ��ṹ��ʽΪCH2=CH-COOH��
(3) ����̼̼������ͬһֱ���ϣ�̼̼˫���ͱ�����ƽ���νṹ���ҵ����ǿ�����ת�ģ����Ը��ݽṹ��ʽ���жϣ����������е�̼ԭ�Ӷ��ǹ���ģ������ϵ���ԭ����1���ǿ��ܹ���ģ�����![]() ������20��ԭ�ӹ��棻(4) �ñ�ȩ��ȡ1��2һ�������������ŷ����仯����������Ŀ�仯��1��2һ��������2���ǻ����Ҵ������ڵ�̼ԭ���ϣ�����Ӧ���Ʊ���ϩ���ȼ��ԭΪ��������Ũ������������·�����ȥ��Ӧ���ñ�ϩ��������ӳɣ��Ƶ�1��2һ������飬Ȼ�����ڼ��ˮ��Һ������ˮ�⣬��1��2һ���������ʺϳ�·�����η����ķ�Ӧ��������˳��Ϊ�ݢ٢ܢ�ݢ٢ܢڻ�ܢ٢ܢڣ���ѡD��(5)������A����ʽΪC5H8�����Ǻϳ���Ȼ�ĵ��壬��Ϊ�����ϩ������A�ϳɵ���Ȼ��˳ʽ�ṹ��ʽΪ��
������20��ԭ�ӹ��棻(4) �ñ�ȩ��ȡ1��2һ�������������ŷ����仯����������Ŀ�仯��1��2һ��������2���ǻ����Ҵ������ڵ�̼ԭ���ϣ�����Ӧ���Ʊ���ϩ���ȼ��ԭΪ��������Ũ������������·�����ȥ��Ӧ���ñ�ϩ��������ӳɣ��Ƶ�1��2һ������飬Ȼ�����ڼ��ˮ��Һ������ˮ�⣬��1��2һ���������ʺϳ�·�����η����ķ�Ӧ��������˳��Ϊ�ݢ٢ܢ�ݢ٢ܢڻ�ܢ٢ܢڣ���ѡD��(5)������A����ʽΪC5H8�����Ǻϳ���Ȼ�ĵ��壬��Ϊ�����ϩ������A�ϳɵ���Ȼ��˳ʽ�ṹ��ʽΪ��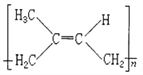 ����6�����ݾ�̼����
����6�����ݾ�̼����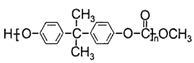 �Ľṹ��ʽ��֪���ϳɾ�̼����������ɫ��ѧԭ��̼�������(
�Ľṹ��ʽ��֪���ϳɾ�̼����������ɫ��ѧԭ��̼�������( )��
)��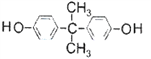 ���Ͼۺ϶��ɡ�
���Ͼۺ϶��ɡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʵ��װ����ȡ�����������ش�����������

(1)��A�Թ��м����Ҵ��������Ũ�����˳����_______________________________��
(2)д��A�Թ��з�����Ӧ�Ļ�ѧ����ʽ___________________________________________
(3)B�Թ�����װҺ��ӦΪ___________�������������ɺ������ڸ���Һ��______(����������������)����������ò�Ʒ�������Ҫ������_________________________________________
(4)��װ�������θ���ܴ��泤��������������ܵ�ĩ�˲���B��Һ���������ڴ˴����θ���ܵ������Т�_________________________________________________________
��_________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ͭ��Ũ��������ڼ��������·������·�Ӧ(��Ӧ����ʽ����ƽ)�� Cu+2H2SO4(Ũ)![]() CuSO4+A��+2H2O��ͨ����������������������⣺
CuSO4+A��+2H2O��ͨ����������������������⣺
(1)A���ʿ��Ե���������γ�����AӦ������______������ţ�
a.����� b.�ǵ���� c.���� d.���������� e.����������
(2)A���ʿ���ʹ����KMnO4��Һ��ɫ���˷�Ӧ��______����д��ѧʽ����ͬ��ʧȥ���ӣ���ԭ������______��
(3)һ������ͭƬ��100mL18mol/L��ŨH2SO4��ַ�Ӧ������÷�Ӧ������ת����0.2mol���ӣ����ɵ�CuSO4������Ϊ______g��������ԭ��Ӧ����������ʵ���Ϊ___________mol��
(4)����Ӧ�����õ�����Һ������Ba��OH��2��Һ��ַ�Ӧ����д���˹��̷����ķ�Ӧ�����ӷ���ʽ��______��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ij��̼�Ͻ�(����̼���ֵ��ʵĻ����)��ij��ѧ��ȤС��Ϊ�˲ⶨ��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ã��г�������ʡ�ԣ���ʵ�鷽������ʵ��̽����
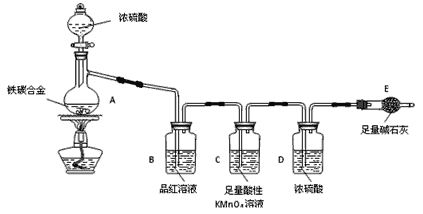
I���ⶨ��������������
��1���������װ�������Ե�һ�ַ����ǣ��رշ�Һ©���Ļ�������Eװ�ú�������һ�����ܣ�Ȼ��___________________________________________________________________����֤��װ�õ����������á�
��2������E������������a g��̼�Ͻ���Ʒ����װ��A�У��ټ���������Ũ���ᣬ��A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����أ�E����b g����̼�Ͻ���������������Ϊ______________________(д����ʽ)��
��3��װ��C������______________________________________________��s5
��4����ͬѧ��Ϊ�����ݴ�ʵ���õ����ݣ�����Ͻ������������������ܻ�ƫ�ͣ�ԭ���ǿ�����CO2��ˮ��������E��ʹb��������Ϊ�Ľ��ķ�����________________________________________��
��5����ͬѧ��Ϊ����ʹ��ͬѧ��Ϊ��ƫ��õ��Ľ������ݴ�ʵ���úϽ���������������Ҳ���ܻ�ƫ�ߡ�����Ϊ���е�ԭ����________________________________________________________��
��̽��Ũ�����ijЩ���ʣ�
��6����A�еμ�������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ�_______________________________________��
��7��A������Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽ��_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ֲ��ӷ����е�һ�ֳɷ֡����ں����ӵ�����˵����������ȷ����

�ٸû��������ڷ�������
�ڷ�����������7��̼ԭ�Ӵ���ͬһƽ�棻
�����IJ���ͬ���칹���ܷ���������Ӧ��
��1mol�û�����������2molBr2������Ӧ��
A. �٢� B. �٢ڢ� C. �ڢ� D. �ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. ����������ͼ��������ﶼ�ǵ����
B. ��NaOH��Һ��μ���FeCl3��Һ���Ʊ�Fe(OH)3����
C. �绯ѧ��ʴ����ɽ�����ʴ����Ҫԭ��
D. ���Ӽ�һ��ֻ���������ӻ������У����ۼ�һ��ֻ�����ڹ��ۻ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA��ʾ�����ӵ�����ֵ������˵����ȷ����
A. �����£�21.0g ��ϩ���ϩ�Ļ�������к��е�̼ԭ����Ϊ1.5NA
B. ���³�ѹ�£�0.1molC8H18�����еĹ��ۼ���ĿΪ2.0 NA
C. 0.1mol�Ҵ���������ȫ��Ӧ���ɶ�����̼ʱת�Ƶ�����Ϊ0.1NA
D. ��״���£�2.24L����������������ԭ����Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ����ˮ��ɹ�ɵô��Σ����γ�NaCl�⣬������MgCl2��CaCl2��Na2SO4�Լ���ɳ�����ʡ��������Ʊ����ε�ʵ�鷽�������������������£�

��1���ڢڲ�������Ŀ���dz�ȥ�����е�________________���ѧʽ����ͬ�����ڢ�������Ŀ���dz�ȥ��Һ��_______________________________��
��2���ڢݲ��������������еõ������ijɷ�������ɳ��BaSO4��Mg(OH)2��CaCO3��________���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��ķ�Ӧԭ�������ӷ���ʽ��ʾ��ȷ����(����)
A. �����£�����Ȼ����ҺpH<7��֤��һˮ�ϰ������NH4+��2H2O===NH3��H2O��H3O��
B. ������������Һ��ȥþ���е���������2Al��2OH����2H2O===2AlO2-��3H2��
C. ��̼��������Һ����ˮ�����е��Ȼ���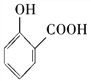 ��2HCO3-��
��2HCO3-�� ��2H2O��2CO2��
��2H2O��2CO2��
D. �ø�����ر���Һ�ζ����2MnO4-��16H����5C2O4-=2Mn2����10CO2����8H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com