
����Ŀ�������й�˵����ȷ�ҽ��ͺ�������
˵�� | ���� | |
A | һ���¶�ѹǿ�£�2 g H2 �� 4 g H2 ��ȫȼ�գ����� ȼ���ȵ���ֵ�� | 4 g H2 �ų������� |
B | 2SO2(g)��O2(g) ƽ����ټ��� SO2��Q ���� | ƽ�����ƣ��ų��������� |
C | ������ʵ���Ũ�ȵ� NaI �� KBr ���Һ�еμ� AgNO3 ��Һ�������ɻ�ɫ AgI ���� | Ksp(AgI)��Ksp(AgBr) |
D | �����ʵ���Ũ�� Na2CO3 �� pH ���� CH3COONa | H2CO3 �����Ա� CH3COOH ǿ |
A.AB.BC.CD.D
���𰸡�C
��������
A. ������������Խ��ȼ�շų���������Խ�࣬4 g H2 �ų������ࣻ��ȼ����ָ����1mol��ȼ����ȫȼ�������ȶ���������ʱ�ų���������һ���¶�ѹǿ�£�������ȼ���ȵ���ֵ�Ǹ���ֵ��A������
B. ��һ����Ӧȷ���Ժ����÷�Ӧ�ġ�H�Ͳ��ٷ����仯��������Ӧ��ƽ����ټ��� SO2��ƽ�����ƣ��ų��������������ǡ�H������B������
C. ������ʵ���Ũ�ȵ� NaI �� KBr���Һ�еμ�AgNO3 ��Һ�������ɻ�ɫ AgI ������˵�������Ӻ��������ȷ�����Ӧ���ɸ����ܵij�����Ksp(AgI)��С������Ksp(AgI)��Ksp(AgBr)��C��ȷ��
D. �����ʵ���Ũ�� Na2CO3 �� pH ���� CH3COONa��˵��Na2CO3��ˮ����������CH3COONa��ˮ������������Խ��Խˮ��Ĺ��ɿ�֪���γɸ��ε���Խ������ˮ��������Խǿ����������H2CO3 �����Ա� CH3COOH ����D������
��������������ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�ӦaA(g)��bB(g)![]() pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����
��1���÷�Ӧ���淴Ӧ��________�ȷ�Ӧ����a��b________p(�>����<������)��
��2����ѹʱ��A����������________(�������С�����䡱����ͬ)������Ӧ����________��
��3��������B(�������)����A��ת����________��B��ת����________��
��4���������¶ȣ���ƽ��ʱ��B��C��Ũ��֮��c(B)/c(C) ��________��
��5�������������ƽ��ʱ��������������ʵ���________��
��6����B����ɫ���ʣ� A��C��Ϊ��ɫ���ʣ������C(�������)ʱ��������ɫ________����ά�������������ѹǿ���䣬��������ʱ����������ɫ________��(���dz����������䡱)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��������һ�����ᣬ���ᾧ��(H2C2O4��2H2O)������ˮ���۵�ϵͣ����Ȼ��ۻ��������ͷֽ⡣����(H2C2O4)���������Ƶķ�Ӧ��H2C2O4+Ca(OH)2=CaC2O4��(��ɫ)+2H2O��
I.ijС��ͬѧ����0.1mol��L-1�IJ�����Һ480ml����֤���������
��1�����ø���Һ��Ҫ���ᾧ��___g��(��֪H2C2O4��2H2O����Է�������Ϊ126)
��2�����в�����ʹ������Һ�����ʵ���Ũ��ƫ�͵�����________��
A.����ʱ��������ƿ�̶���
B.��ˮʱ�����̶��ߣ��ý�ͷ�ι�����
C.ת�ƹ���������������Һ����
D.��������ʱ�������������
E.����ƿ������ˮϴ�Ӹɾ���û�и����������������Һ
F.���ù����У�δ������ˮϴ���ձ��Ͳ�����
��3��ȡһ����������Һװ���Թܣ�����һ����������Ը��������Һ�����Թܣ�������Һ��ɫ(MnO4-����ԭΪMn2+)����Ӧ�����ӷ���ʽΪ___���÷�Ӧ�ķ�Ӧ��������������Ҫԭ�������___��
II.���ݲ��ᾧ�����ɣ����������ȷֽ����ΪCO��CO2��H2O��ͨ����ͼװ����֤���ᾧ��IJ��ַֽ���
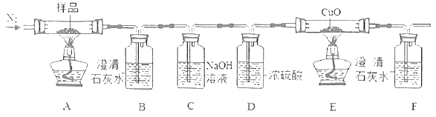
��4����Ӧ��ʼǰͨ�뵪����Ŀ����___��
��5��B�г���ʯ��ˮ����ǣ�������֤��������һ����CO2��������___��
��6��E�й����ɺ�ɫ��F�г���ʯ��ˮ����ǣ�˵�������к�������___ (�ѧʽ)��
��7��������װ��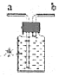 �ռ������CO������Ӧ��___�˽���(ѡ����a������b��)��
�ռ������CO������Ӧ��___�˽���(ѡ����a������b��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ��Ԫ�ء�
A | |||||||||||||||||
B | C | D | E | F | T | ||||||||||||
G | H | I | J | K | L | ||||||||||||
M | N | O | |||||||||||||||
�Իش��������⣨ע�⣺ÿ���е���ĸ����Ϊ�ϱ��е���ĸ���ţ�����ΪԪ�ط��ţ�
(1)N�ĵ��ʺ�ˮ������Ӧ�����ɹ���X����I�ĵ�����X��Ӧ�Ļ�ѧ����ʽ_______��
(2)D����̬�⻯���VSEPRģ�͵�����Ϊ_______��
(3)��A��C��D�γɵ�ACD�����У�����������������= _______________��
(4)Ҫ֤��̫�����Ƿ���R Ԫ�أ��ɲ��õķ�����__________________________��
(5)Ԫ��M�Ļ�����(ME2L2)���л��ϳ��п������������Ȼ��������������л��ﷴӦ���ش����⣺
��ME2L2������Ϊ���ɫҺ�壬����CCl4��CS2�Ȼ��ܣ��ݴ˿��ж�ME2L2��_________������ԡ��Ǽ��ԡ������ӡ�
�ڽ�N��O�ĵ����õ������Ӻ����D������������Ӧ��ˮ����Ũ��Һ�У����Ƴ�ԭ��أ�����ɸ������ϵ�Ԫ�ص���Χ���ӹ����ʾʽΪ______________________��
(6)��O2����Һ�м��백ˮ���γ���ɫ�������������백ˮ���������ܽ�����ɫ����Һ��д�������ܽ�����ӷ���ʽ_____��
(7)��F ��K����Ԫ���γɵĻ�����������ԭ�ӵļ۵���ȫ������ɼ�����û�����Ŀռ乹�͵�����Ϊ___��
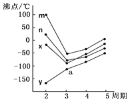
(8)��ͼ�������߷ֱ��ʾ��A�塢��A�塢��A�塢��A��Ԫ����̬�⻯��е�仯����E���⻯�����ڵ�������__����m��n��x��y����
(9)1183 K���´�N����Ļ����ṹ��Ԫ��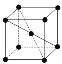 ��ʾ��1183 K����ת��Ϊ
��ʾ��1183 K����ת��Ϊ ��ʾ�ṹ�Ļ����ṹ��Ԫ����1183 K���µľ����У��ռ�������Ϊ____����1183 K���ϵľ����У���Nԭ�ӵȾ����������Nԭ����Ϊ____������ѻ���ʽ������Ϊ_____��
��ʾ�ṹ�Ļ����ṹ��Ԫ����1183 K���µľ����У��ռ�������Ϊ____����1183 K���ϵľ����У���Nԭ�ӵȾ����������Nԭ����Ϊ____������ѻ���ʽ������Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֬������֬�����ܳƣ����Ƕ��ָ�֬����ĸ���������֬������Ҫ��Ӫ�����ʣ�������Ҫ�Ļ���ԭ�ϡ�������������֬���е�̼̼�����ͼ���![]() ���йص��ǣ� ��
���йص��ǣ� ��
A.�·��ϵ���֬��������ϴȥ
B.����������֬���������������ն���֬����ά���غͺ��ܲ���
C.ֲ����ͨ���⻯��������ֲ�����ͣ��������ͣ�
D.֬�����л�����֯�ﴢ����������Ҫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D��E�������������������ʣ��ٸ�ȡ0��1mol�ֱ���ȼ�գ�����B��C��Eȼ�����õ�CO2��Ϊ4��48L����״������A��Dȼ�����õ�CO2����������������3�����������������£�A��B��C���ܸ����������ӳɷ�Ӧ������A����ת��ΪD��B����ת��ΪC��C����ת��ΪE����B��C����ʹ��ˮ������KMnO4��Һ��ɫ����A��D��E�����ʣ�������м������ʱ��A�����巢��ȡ����Ӧ��
(1) д��D��E�Ľṹ��ʽ��D__________E__________��
(2) д��A��Һ�巴Ӧ�Ļ�ѧ����ʽ______________________________________
(3) д��ʵ������ȡB�Ļ�ѧ����ʽ______________________________________
(4) д��C�����Ӿ۷�Ӧ����Ľṹ��ʽ_________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CCuS��һ�ֶ�����̼�IJ�����������ļ��������ּ����ɽ�CO2��Դ���� ��������Ч�档CO2��������������ɵ�̼�л����Ҫ�����·�Ӧ��
��ӦI��CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H1=��49.8kJ��mol��1
CH3OH(g)��H2O(g) ��H1=��49.8kJ��mol��1
��ӦII��CH3OCH3(g)��H2O(g) ![]() 2CH3OH(g) ��H2=��23.4kJ��mol��1
2CH3OH(g) ��H2=��23.4kJ��mol��1
��ӦIII��2CO2(g)��6H2(g) ![]() CH3OCH3(g)��3H2O(g) ��H3
CH3OCH3(g)��3H2O(g) ��H3
��1����H3=_____________kJ��mol��1
��2�����º��������£����ܱ�������ͨ�˵����ʵ�����CO2��H2��������ӦI������������˵����ӦI�ﵽƽ��״̬����_______(����ţ���
A.�����ڵĻ��������ܶȱ��ֲ���B.��Ӧ��ϵ��ѹǿ���ֲ���
C.CH3OH��CO2��Ũ��֮�ȱ��ֲ���D.����3NA��H-O��ͬʱ����2NA��C=O��
��3����ӦII��ij�¶��µ�ƽ�ⳣ��Ϊ0.25,���¶��£����ܱ������м��˵����ʵ����� CH3OCH3(g)��H2O(g)����Ӧ��ijʱ�̲�ø����Ũ�����£�
���� | CH3OCH3(g) | H2O(g) | CH3OH(g) |
Ũ��/mol��L��1 | 1.6 | 1.6 | 0.8 |
��ʱ![]() ___
___![]() ������>������<������=����������Ӧ�ﵽƽ��״̬ʱ�����������CH3OH ������� V(CH3OH)%= _____%��
������>������<������=����������Ӧ�ﵽƽ��״̬ʱ�����������CH3OH ������� V(CH3OH)%= _____%��
��4����ijѹǿ�£���ӦIII�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ƽ��ת������ͼ��ʾ��T1�¶��£���6 mol CO2��12 mol H2����3 L���ܱ������У�10 min��Ӧ�ﵽƽ��״̬����0-10 min�ڵ�ƽ����Ӧ����V(CH3OCH3)=____��
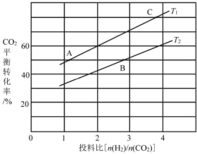
��5����ѹ�½�CO2���ϰ������1 ��3��ϣ��� ��ͬ���������·�����ӦI�ͷ�ӦIII������ͬ��ʱ�����CH3OH��ѡ���ԺͲ������¶ȵı仯����ͼ��
���У�CH3OH��ѡ����=![]() ��100%
��100%
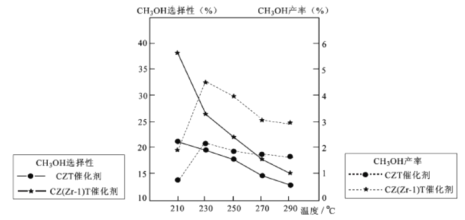
���¶ȸ���230����CH3OH�������¶����߶��½���ԭ����________��
�������������ºϳɼ״��Ĺ�ҵ������_________��
A. 230�� B. 210�� C.���� CZT D.���� CZ(Zr-1)T
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ����101kPaʱ��O2(g) +2H2(g) = 2H2O(l) ��H = ��483.6 kJ��mol��1 ��ϡ��Һ�У�OH��(aq)+ H+(aq) = 2H2O(l) ��H = ��57.3 kJ��mol��1����֪��H2��O2�������巴Ӧ����1molҺ̬ˮ������1mol��̬ˮ��ų�44kJ���������н�������ȷ���ǣ� ��
A.1mol H2��ȫȼ������Һ̬ˮ���ų�������Ϊ285.8 kJ
B.H2��O2��Ӧ����Һ̬ˮʱ���Ȼ�ѧ����ʽΪ![]() O2(g) +H2(g) = H2O(l) ��H = ��571.6 kJ��mol��1
O2(g) +H2(g) = H2O(l) ��H = ��571.6 kJ��mol��1
C.ϡ������ϡNaOH��Һ��Ӧ���к���Ϊ��57.3 kJ
D.ϡ������ϡNaOH��Һ��Ӧ����1molˮ���ų�57.3 kJ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£������Ϊ5L���ܱ������У�����1mol A���壬�������¿��淴Ӧ��2A(g)![]() B(g)+C(?)��2min��Ӧ�ﵽƽ�⣬AΪ0.4mol�����ı䷴Ӧ����ʱ������ͼ�ı仯��P0��ʾ1������ѹ���������������в���ȷ����
B(g)+C(?)��2min��Ӧ�ﵽƽ�⣬AΪ0.4mol�����ı䷴Ӧ����ʱ������ͼ�ı仯��P0��ʾ1������ѹ���������������в���ȷ����
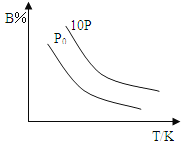
A.0~2minʱ����B���ʵ�ƽ����Ӧ����Ϊ0.03mol/(L��min)
B.�÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ����CΪ����������
C.�ﵽƽ������¶Ⱥ�����������䣬�ٳ���1mol A��ƽ��������Ӧ�����ƶ�
D.���¶Ⱥ�����������䣬��ʼʱ����B��C��0.5mol����ﵽƽ��ʱ��n(A)С��0.4mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com