
����Ŀ��Ԫ��X��Y��Z��W��M��Nԭ��������������X��M��W��N�ֱ�ͬ���壬��Ԫ��X��Y��Z��W�������������ڣ���������ԭ������֮��Ϊ22������������֮��Ϊ16����ش��������⣺
(1)д��Ԫ������ M_________________��N_____________________��
(2)д��M2W2�ĵ���ʽ________��Z2X4�ṹʽ__________________________��
(3)X��Z��W�γɵĻ������п��������ʵ���������ѧ����������________��
(4)ZX3��X2W���ܽ�Ⱥܴ��ԭ����Ҫ��__________________________________________��
(5)�����������������Z2X4��ȼ��X2W2����ȼ�������ﻷ������Ⱦ��д�����߷�Ӧ�Ļ�ѧ����ʽ_________��
(6)����X��W��M��N����Ԫ����ɵ����ֻ��������Ӧ���д̼�����ζ����ų�����������ͨ�����ᱵ��Һ�е����ӷ���ʽ________________________��
���𰸡��� �� ![]()
![]() ���Ӽ����ۼ� NH3��H2O�����м��ԣ���NH3���Ӻ�H2O����֮������γ�������������� N2H4+ 2H2O2=N2+4H2O 3Ba2+ +3SO2+ 2NO3-+2H2O=3BaSO4��+2NO+4H+
���Ӽ����ۼ� NH3��H2O�����м��ԣ���NH3���Ӻ�H2O����֮������γ�������������� N2H4+ 2H2O2=N2+4H2O 3Ba2+ +3SO2+ 2NO3-+2H2O=3BaSO4��+2NO+4H+
��������
Ԫ��X ��Y��Z��W��M��Nԭ��������������W��Nͬ���壬���ԭ��������֪��W���ڵڶ����ڡ� N���ڵ������ڣ���Ԫ��X��Y��Z��W�������������ڣ���Xֻ��ΪHԪ�أ� Y��Z���ڵڶ����ڣ� X��Y��Z��W��ԭ������֮��Ϊ22 ������������֮��Ϊ16����Y��Z��Wԭ������֮��Ϊ21������������֮��Ϊ15������֪YΪCԪ�ء�ZΪNԪ�ء�WΪOԪ�أ���NΪSԪ�أ�X��Mͬ���壬Mԭ����������0����MΪNa���ݴ˽��
(1)Ԫ�����ƣ�MΪ�ƣ�NΪ��
(2)M2W2ΪNa2O2�������ʽΪ![]() ��Z2X4ΪN2H4���ṹʽ
��Z2X4ΪN2H4���ṹʽ![]() ��
��
(3)X��Z��W�γɵĻ������п��������ʵ���ΪNH4NO3��������ѧ�������������Ӽ����ۼ���
(4)ZX3��X2W���ܽ�Ⱥܴ��ԭ����Ҫ��NH3��H2O�����м��ԣ���NH3���Ӻ�H2O����֮������γ�������������ܣ�
(5)�����������������N2H4��ȼ�ϡ�H2O2����ȼ��,���ﻷ������Ⱦ,��Ӧ���ɵ�����ˮ,���߷�Ӧ�ķ���ʽΪ:N2H4+ 2H2O2=N2+4H2O��
(6)����X��W��M��N����Ԫ����ɵ����ֻ�����NaHSO4��NaHSO3���Ӧ���д̼�����ζ����SO2�ų�����SO2����ͨ�����ᱵ��Һ�з�Ӧ�������ᱵ��һ����������Ӧ�����ӷ���ʽΪ3Ba2+ +3SO2+ 2NO3-+2H2O=3BaSO4��+2NO+4H+��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ij��ȤС��Ժ�����ɳ�Ĵ��ν����ᴿʵ�������ͼ����ش��������⡣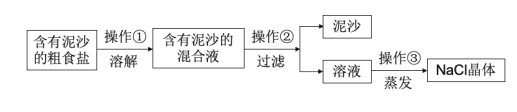
��1�����������õ��IJ���������__��
��2��������������������__��
��3����ʵ������NaCl�IJ���ƫ�ͣ�����ܵ�ԭ��__����ѡ���
A������ʱ��ֽ������
B������ʱû���ò���������
C���ܽ⺬��ɳ�Ĵ���ʱ������ˮ������
D�������Ȼ��ƾ���û�к�ɣ�����ˮ��
E�����˺����ֽ��ʪ�ģ�ֽ�ϵ�ˮ�ܽ���һЩ�Ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���̷����壨FeSO4��7H2O��M=278g/mol��������ȱ����ƶѪҩƷ����Ҫ�ɷ֡�ʵ�����������᳧����������Ҫ�ɷ�ΪFe2O3������FeS��SiO2�����Ʊ��̷��Ĺ������£�
 �Իش𣺣�1��������Ϊ_____________����д�������ƣ���
�Իش𣺣�1��������Ϊ_____________����д�������ƣ���
��2�� �Լ�Y����ҺX��Ӧ�����ӷ���ʽΪ__________________________________��
��3�����������̷������к���Fe2+��ʵ�������________________________________��
��4���������˳������Ϊ��_______________����ȴ�ᾧ������ ��__________�����
��5��ijͬѧ������KMnO4��Һ�ⶨ�̷���Ʒ��Fe2+������
a.��ȡ11.5g�̷���Ʒ���ܽ⣬���Ƴ�1000mL��Һ��
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
�ٲ���a������Һʱ��Ҫ�IJ�������������������Ͳ���ձ�����ͷ�ι��⣬����___________��
�ڸ�ͬѧ��Ƶ����еζ���ʽ�����������____________(�гֲ�����ȥ)(����ĸ���)��
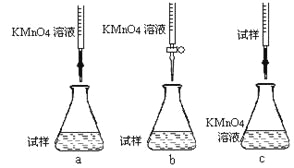
�۵ζ�ʱ������Ӧ�����ӷ���ʽΪ��_______________________________________��
���жϴ˵ζ�ʵ��ﵽ�յ�ķ�����_____________________________�����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ������KMnO4��ҺҺ�棬������������ȷ����ʹ�ⶨ���________������ƫ������ƫ��������Ӱ��������
�ݼ���������Ʒ��FeSO4��7H2O����������Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ſ�ѧ�����ķ�չ�������ӵ������IJⶨ�ֶ�Խ��Խ�࣬�ⶨ��ȷ��ҲԽ��Խ�ߣ�����һ�ּ��еIJⶨ���������岽��Ϊ��
(1)������NaCl��ϸ�������ȷ��ȡmgNaCl���岢ת�Ƶ���������A�С�
(2)�õζ�����A�����еμӱ��������������ӱ���A�����Ŀ̶��ߣ������NaCl��������ΪVcm3��
�ٲ���(1)��A���������__________���������ƣ���
�ڲ���(2)������ʽ�ζ��ܺû��Ǽ�ʽ�ζ��ܺã�__________��������______________��
���ܷ��ý�ͷ�ιܴ��沽��(2)�еĵζ���__________��������____________________��
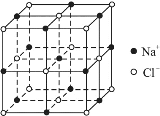
����֪NaCl����Ľṹ����ͼ��ʾ����X���߲��NaCl�����п��������Na+��Cl-���ƽ������Ϊacm�����������ⶨ������ð����ӵ�����NA�ı���ʽΪ��NA=______mol-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ�أ�Se���ǵ�4���ڢ�A��Ԫ�أ������к����������ͻ��������������õİ뵼����ϣ�����������������ڹ������µ����Կ���߽�ǧ����������������ܡ���ش��������⣺
��1����̬Seԭ�Ӽ۵��ӵĹ����ʾʽΪ__��
��2��As��Se��ͬһ����Ԫ�أ�As�ĵ�һ�����ܱ�Se��ԭ����___��
��3�����ڿ�����ȼ��������SeO2�������£�SeO2���ӷ��İ�ɫ���壬�۵�Ϊ340~350�棬315��ʱ��������SeO2������___���壻д��һ����SeO2��Ϊ�ȵ�����������ӵĻ�ѧʽ__��
��4��H2SeO4��H2SO4���ƣ���һ�ֲ��ӷ���ǿ�ᡣSeO42-�Ŀռ乹��Ϊ___������ԭ�ӵ��ӻ���ʽΪ___��
��5������п��һ����Ҫ�İ뵼����ϣ��侧���ṹ��ͼ��ʾ��Znԭ�ӵ���λ��Ϊ___�����þ����ܶ�Ϊ��g��cm-3������п��Ħ������ΪMg��mol-1����NA���������ӵ�������������a���������ı߳���Ϊ___nm��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��������NiO�����оʹ�������ͼ��ʾ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ�������������Գʵ����ԣ����˻�������Ni��O�ı�ֵȴ�����˱仯��ij��������Ʒ����и�����Ni��O��97��100
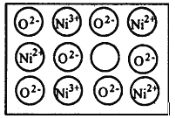
(1)д������������Ʒ�Ļ�ѧʽ_________________��
(2)�Լ��㾧����Ni2+��Ni3+�����Ӹ���֮��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�����Һ�п��ܴ������е��������±���ʾ��
������ | H����K����Al3����NH4+��Mg2�� |
������ | Cl����OH����CO32-��AlO2- |
Ϊ̽����ɷ֣�ijͬѧ��Na2O2���뵽���������Һ�в��ȣ�������������������ʵ��������Na2O2�����ʵ����Ĺ�ϵ�ֱ���ͼ��ʾ��

(1)����Һ��һ�����е���������________________________________�����Ӧ���ʵ���Ũ��֮��Ϊ ____________����Һ��һ�������ڵ���������_______________________��
(2)д���������ٵ����ӷ���ʽ ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ں����ܱ������з����Ŀ��淴ӦN2(g)��3H2(g)![]() 2NH3(g) ��H<0����˵����Ӧ�ﵽ��ѧƽ��״̬��Ϊ�� ��
2NH3(g) ��H<0����˵����Ӧ�ﵽ��ѧƽ��״̬��Ϊ�� ��
A.�Ͽ�һ��N��N����ͬʱ��6��N��H������
B.���������ܶȲ���
C.��������ƽ����Է�������
D.N2��H2��NH3��������Ϊ1��3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о�![]() ��
��![]() ��
��![]() �ȵĴ��������Ի�����������Ҫ���塣
�ȵĴ��������Ի�����������Ҫ���塣
��1����ѧ�������о����ô�������β���е�NO��COת���![]() ��
��![]() ���䷴ӦΪ��
���䷴ӦΪ��![]()
![]()
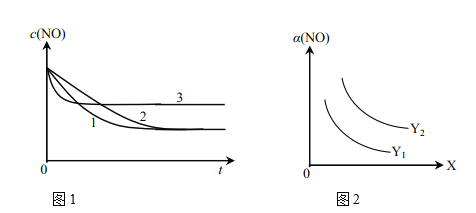
��Ϊ���о���������Ը÷�Ӧ��Ӱ�죬�����±�����ʵ�飬��ò�ͬʱ��NO��Ũ�ȣ�c����ʱ��仯��������ͼ1��ʾ��1��2��3������ʵ����������________������֪��ʹ�õ���������ʱ����������ȱ���������ѧ��Ӧ���ʡ���
ʵ���¶�NO��ʼŨ��O��ʼŨ�ȴ����ȱ��������������ţ��棩
ʵ�� ��� | �¶� ���棩 | NO��ʼŨ��
| CO��ʼŨ��
| �����ȱ����
| �������� ��g�� |
�� | 280 |
|
| 82 | 50 |
�� | 280 |
|
| 124 | 50 |
�� | 350 |
|
| 124 | 50 |
��ͼ2��ʾNO��ƽ��ת���ʣ�a�����¶ȡ�ѹǿ�仯��ʾ��ͼ��X��ʾ����________��������________��Y��ʾ����________����Y1________Y2������>������<������
��2��һ���¶��£���![]() ��
��![]() �������1��2�����ܱ������з�����Ӧ
�������1��2�����ܱ������з�����Ӧ![]() ���ﵽƽ��ʱ
���ﵽƽ��ʱ![]() ���������Ϊ25�����÷�Ӧ��ƽ�ⳣ��
���������Ϊ25�����÷�Ӧ��ƽ�ⳣ��![]() ________��
________��
��3������ԭ��ط�Ӧ��ʵ��![]() ���������ܷ�ӦΪ
���������ܷ�ӦΪ ���������ҺΪ���ԡ�����һ��ʱ��õ�ظ�����������ҺpH________�����������������С���������������������缫��ӦʽΪ________��
���������ҺΪ���ԡ�����һ��ʱ��õ�ظ�����������ҺpH________�����������������С���������������������缫��ӦʽΪ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com