
����Ŀ��ij�Ͻ�ֻ����������ͭ����Ԫ�أ�ȡ�Ͻ�![]() �����뵽��
�����뵽��![]() ϡ�������Һ�У�ǡ����ȫ��Ӧ���ų���ɫ����11.2L����״���£�������Ӧ�����Һ�ֳ�2�ȷݣ���һ����Һ�еμ�
ϡ�������Һ�У�ǡ����ȫ��Ӧ���ų���ɫ����11.2L����״���£�������Ӧ�����Һ�ֳ�2�ȷݣ���һ����Һ�еμ�![]() ����������������һ����Һ�еμӰ�ˮ�����������˵ó���bg��������յù���cg��������5.4g���ټ��뵽������
����������������һ����Һ�еμӰ�ˮ�����������˵ó���bg��������յù���cg��������5.4g���ټ��뵽������![]() ��Һ�У�ʣ�����dg������˵������ȷ����
��Һ�У�ʣ�����dg������˵������ȷ����
��֪��![]() ����
����![]() �������ɫ������
�������ɫ������
A. ![]()
B. ԭ�Ͻ���ͭ������Ϊ![]()
C. ��������![]() ϡ������μ��뵽�Ͻ��У��ų�������Ϊ
ϡ������μ��뵽�Ͻ��У��ų�������Ϊ![]() ����״���£�
����״���£�
D. d����һȷ��ֵ������Ϊ8
���𰸡�D
��������
����Ԫ�ػ�����֪ʶ�����жϳ���ɫ����ΪNO��һ����Һ�еμ�![]() ��Һ��������������
��Һ��������������![]() ��������ҺΪ
��������ҺΪ![]() �Ļ��Һ������һ����Һ�еμӰ�ˮ���������õ�����
�Ļ��Һ������һ����Һ�еμӰ�ˮ���������õ�����![]() Ϊ
Ϊ![]() ��
��![]() ��
��![]() ��ת��Ϊ
��ת��Ϊ![]() �����˺������Һ������cgΪ
�����˺������Һ������cgΪ![]() ��
��![]() ��������dgΪ
��������dgΪ![]() ���ݴ˷�������
���ݴ˷�������
A. �Ͻ���뵽������Һ���ݵ�ʧ�����غ㣬�Ͻ��ܹ�ʧȥ����![]() ����Ӧ����Һ�������Ӵ������
����Ӧ����Һ�������Ӵ������![]() ,������Һ�������
,������Һ�������![]() ������
������![]() ��A����ȷ��
��A����ȷ��
B. ![]() ,
,![]() ,
,![]() ������
������![]() ��
��![]() ��HԪ�ص����ʵ���֮�͵���
��HԪ�ص����ʵ���֮�͵���![]() ���ɵ�
���ɵ�![]() ,ԭ������
,ԭ������![]()
![]() ��B����ȷ��
��B����ȷ��
C. ������Һ�в���![]() ��Ϊ
��Ϊ![]() �Ļ��Һ������������������������
�Ļ��Һ������������������������![]() ϡ������μ��뵽�Ͻ��У�������
ϡ������μ��뵽�Ͻ��У�������![]() ����
����![]() ��������������������������������������
��������������������������������������![]() �����ݵ�ʧ�����غ����ɵ�NO������Ϊ
�����ݵ�ʧ�����غ����ɵ�NO������Ϊ![]() ��C����ȷ��
��C����ȷ��
D. ���ʣ�����dgΪ![]() 8g����һȷ��ֵ��D�����
8g����һȷ��ֵ��D�����
��ѡD��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼװ�ý���ʵ�飬���ش��������⣺

��1���ж�װ�õ����ƣ�A��Ϊ___________ ��B��Ϊ______________��
��2��п��Ϊ________�����缫��ӦʽΪ___________________________________��ͭ��Ϊ________�����缫��ӦʽΪ___________________________________��ʯī��C1Ϊ______�����缫��ӦʽΪ__________________________________��ʯī��C2����������ʵ������Ϊ______________________________________��
��3����C2������224mL���壨��״̬��ʱ��п�������仯_________���������С����________g��CuSO4��Һ������_________�����ӡ��������٣�_________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ijͬѧ��������ƽ�����ձ�����������ƽƽ����״̬��ͼ����ͼ�п��Կ�������ͬѧ�ڲ���ʱ��һ��������__���ձ���ʵ������Ϊ__����С����Ϊ5g����

��2����֪ij��84������Һƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84������Һͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺
��Ч�ɷ� | NaClO |
��� | 1000mL |
�������� | 25% |
�ܶ� | 1.19g��cm-1 |
�ٸá�84������Һ���ʵ���Ũ��Լ____ mol��L-1(����һλС��)��
��ijͬѧȡ20mL�á�84������Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na+)=__mol��L-1��
�ۡ�84������Һ��ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��Ũ��������ϡ����������ǿ��84������Һ���������������в���ʹ�����Ƶ�������ҺŨ��ƫ�͵���__��
a����ȡŨ����ʱ��������Ͳ�̶��� b��δ����ȴ�ͽ���Һת��������ƿ
c����Һ����ҡ�Ⱥ����������� d��û��ϴ����ȡŨ�������Ͳ
e������ʱ��������ƿ�̶��� f������ƿ�ڲ���һ��������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£����ڿ��淴ӦX(g)+3Y(g)![]() 2Z(g)����X��Y��Z����ʼŨ�ȷֱ�Ϊc1��c2��c3(����Ϊ��)����ƽ��ʱ��X��Y��Z��Ũ�ȷֱ�Ϊ0.1 mol/L��0.3 mol/L��0.08 mol/L���������ж���ȷ���� (�� ��)
2Z(g)����X��Y��Z����ʼŨ�ȷֱ�Ϊc1��c2��c3(����Ϊ��)����ƽ��ʱ��X��Y��Z��Ũ�ȷֱ�Ϊ0.1 mol/L��0.3 mol/L��0.08 mol/L���������ж���ȷ���� (�� ��)
A. c1��c2��3��1 B. ƽ��ʱ��Y��Z����������֮��Ϊ2��3
C. X��Y��ת���ʲ���� D. c1��ȡֵ��ΧΪ0 mol/L<c1 <0.14 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E ��Ϊ������Ԫ�أ�A ԭ��ʧȥһ�����Ӻ��Ϊһ�����ӣ�C ��-1 ������������ԭ�Ӿ�����ͬ�ĵ��Ӳ�ṹ��D��C ����һ���ڣ�D����ͬ���ڵ�B�γ� BD2�����ӻ����E �� CΪͬһ����Ԫ�أ�������������Ӧ��ˮ����Ϊһ��ǿ�ᡣ�����������Ϣ�ش��������⡣
��1��BԪ����Ԫ�����ڱ��е�λ����____________��
��2��A�� E �γɵ��������ĵ���ʽΪ____________��
��3��C���⻯���� D���⻯��ķе���Ƚϣ� ____________(�û�ѧʽ��ʾ�����ã��������ӣ��� ����ԭ��______________��
��4��E ���⻯���� E ������������Ӧˮ���ﷴӦ���������к��еĵĻ�ѧ����____________��
��5��E ������������Ӧ��ˮ�����ϡ��Һ��������۷�����Ӧ�����ӷ���ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ���������Ϻͺϳ�����Ҫ�����л�ԭ�ϣ��ұ������ⷨ��Ŀǰ��������������ϩ����Ҫ�������仯ѧ����ʽΪ��
![]()
��1���������¶ȣ��÷�Ӧ��ƽ�ⳣ�������H_______0������ڡ���С�ڡ������÷�Ӧ��_______________���������Է����С�
��2��ά����ϵ��ѹǿ�Ѻ㶨�����¶�Tʱ�����ʵ���Ϊ2mol�����Ϊ1L���ұ��������������ⷴӦ����֪�ұ���ƽ��ת����Ϊ80%�����ڸ��¶��·�Ӧ��ƽ�ⳣ��K=_____��
��3�������Ϊ2L�ĺ����ܱ�������ͨ��2mol�ұ�������2���Ӻ�ﵽƽ�⣬���������Ũ����0.5mol/L�����ұ������ķ�Ӧ����Ϊ_________________��ά���¶Ⱥ�����������䣬������ƽ������ͨ��1mol������1mol�ұ���������v��_______v�棨����ڡ�����С�ڡ����ڡ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ѧ����������װ��ͼ�ɹ���ʵ����CO2��H2O�ϳ�CH4�����������������(����)
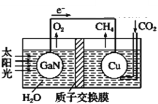
A. ��ع���ʱ��ʵ���˽�̫����ת��Ϊ����
B. ͭ�缫Ϊ�������缫��ӦʽΪCO2+4e-+8H+=CH4+2H2O
C. ����� H+ �����ӽ���Ĥ���������ƶ�
D. Ϊ��߸��˹����ϵͳ�Ĺ���Ч�ʣ�����װ���м�������ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±��Dz�ͬ�¶���ˮ�����ӻ��������Իش��������⣺
�¶�(��) | 25 | t1 | t2 |
ˮ�����ӻ����� | 1��10-14 | �� | 1��10-12 |
(1)��25��t1��t2������______(������������������=��)1��10-14��
(2)25���£�ijNa2SO4��Һ��c(SO42-)=5��10-4mol��L-1��ȡ����Һ1mL����ˮϡ����10mL����ϡ�ͺ���Һ��c(Na+)��c(OH-)=________��
(3)25���£���pH=1��������Һ��pH=5��������Һ�������ϣ�����Һ��ˮ�������c(OH-)=________��
(4)��t2���¶���pH=11�Ŀ�������Һa L��pH=1��ϡ������ҺbL��ϣ����û����ҺpH=2����a��b=______������Һ������Ũ�ȵ��ɴ�С������˳����______________________________��(���Ͼ�������Һ���ǰ������ı仯)
(5)��t2���¶���pH=9�Ŀ�������Һ��pH=y��ϡ������Һ�������ϣ����û����Һ�����ԣ���y____3(������������С��������������)������Һ����Ũ�ȵ��ɴ�С������˳����________________________��(���Ͼ�������Һ���ǰ������ı仯)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ͬ��������һ��ʢ��O2����һ��ʢ��N2����ͬ��ͬѹ���������ڵ�����һ��������ͬ�ģ� ��
A.�ܶ�B.������C.ԭ������D.����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com