
ЎҫМвДҝЎҝёҙәПЗв»ҜОпҝЙЧчОӘҙўЗвәН№ММеөзҪвЦКІДБПЈ¬ФЪДЬФҙУлІДБПБмУтөГөҪБЛЙоИлөДСРҫҝЎЈ
Из:ўЩMg(NH2)2 ўЪNaNH2 ўЫ H3NЈӯBH3 ўЬNaAlH4 ўЭLi3AlH6
(1)ёҙәПЗв»ҜОпЙэОВјУИИҝЙЦрІҪ·ЦҪв·ЕіцЗвЖшЈ¬АнВЫЙПөҘО»ЦКБҝөДЙПКцёҙәПЗв»ҜОпЖдҙўЗвДЬБҰЧоөНөДКЗ__________(МоұкәЕ)ЎЈ
(2)ФЪMg(NH2)2әНNaNH2ЦРҫщҙжФЪNH2ЈӯЈ¬NH2ЈӯөДҝХјд№№РНОӘ_________Ј¬ЦРРДФӯЧУөДФУ»Ҝ·ҪКҪОӘ____________ЎЈ
(3)H3NЈӯBH3УлЛ®·ҙУҰЙъіЙТ»ЦЦСОәНH2өД»ҜС§·ҪіМКҪЈә_____________________ЎЈРҙіц»щМ¬BФӯЧУөДјЫөзЧУ№мөАұнҙпКҪЈә__________________________ЎЈ
(4)Li3AlH6ҫ§МеөДҫ§°ыІОКэОӘaЈҪbЈҪ801.7 pmЈ¬cЈҪ945.5 pmЈ¬ҰБЈҪҰВЈҪ90ЎгЎўҰГЈҪ120ЎгЈ¬Ҫб№№ИзНјЛщКҫЈә
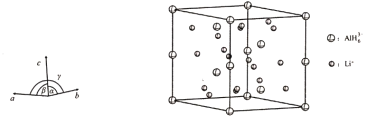
ўЩТСЦӘAlH63ЈӯөД·ЦКэЧшұкОӘ(0Ј¬0Ј¬0)Ўў(0Ј¬0Ј¬![]() )Ј¬(
)Ј¬(![]() Ј¬
Ј¬![]() Ј¬
Ј¬![]() )Ј¬(
)Ј¬(![]() Ј¬
Ј¬![]() Ј¬
Ј¬![]() )Ј¬(
)Ј¬(![]() Ј¬
Ј¬![]() Ј¬
Ј¬![]() )әН(
)әН(![]() Ј¬
Ј¬![]() Ј¬
Ј¬![]() )Ј¬ҫ§°ыЦРLiЈ«өДёцКэОӘ____________ЎЈ
)Ј¬ҫ§°ыЦРLiЈ«өДёцКэОӘ____________ЎЈ
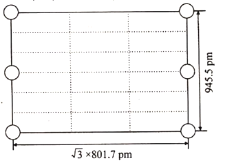
ўЪИзНјКЗЙПКцLi3AlH6ҫ§°ыөДДіёцҪШГжЈ¬№Іә¬УР10ёцAlH63ЈӯЈ¬ЖдЦР6ёцТСҫӯ»ӯіц(НјЦРөДЎр)Ј¬ЗлФЪНјЦРУГЎрҪ«КЈУаөДAlH63Јӯ»ӯіц____________ЎЈ
ўЫҙЛҫ§МеөДГЬ¶ИОӘ____gЎӨcmЈӯ3(БРіцјЖЛгКҪЈ¬ТСЦӘ°ў·ьјУөВВЮіЈКэФјОӘ6.02ЎБ1023molЈӯ1)ЎЈ
Ўҫҙр°ёЎҝўЪ VРО sp3ФУ»Ҝ H3NЈӯBH3+2H2O = NH4BO2 +3H2 ![]() 18
18  »т
»т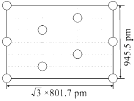
![]()
ЎҫҪвОцЎҝ
(1)АнВЫЙПөҘО»ЦКБҝёҙәПЗв»ҜОпЖдҙўЗвДЬБҰјҙОӘёҙәПЗв»ҜОпЦРЗвФӘЛШөДЦКБҝ·ЦКэЈәўЩMg(NH2)2ЦРHЈҘОӘ![]() ЎўўЪNaNH2ЦРHЈҘОӘ
ЎўўЪNaNH2ЦРHЈҘОӘ![]() ЎўўЫ H3NЈӯBH3ЦРHЈҘОӘ
ЎўўЫ H3NЈӯBH3ЦРHЈҘОӘ![]() ЎўўЬNaAlH4ЦРHЈҘОӘ
ЎўўЬNaAlH4ЦРHЈҘОӘ![]() ЎўўЭLi3AlH6ЦРHЈҘОӘ
ЎўўЭLi3AlH6ЦРHЈҘОӘ![]() Ј¬ЛщТФҙўЗвДЬБҰЧоөНөДКЗўЪЈ»
Ј¬ЛщТФҙўЗвДЬБҰЧоөНөДКЗўЪЈ»
(2) NH2ЈӯөДјЫІгөзЧУКэОӘ![]() Ј¬ёщҫЭVSEPRАнВЫЈ¬NH2ЈӯөДАнВЫ№№РНОӘХэЛДГжМеЈ¬Ттә¬БҪ¶Ф№В¶ФөзЧУЈ¬ЛщТФNH2ЈӯөДҝХјд№№РНОӘVРОЈ¬ТтјЫІгөзЧУ¶ФКэОӘ4Ј¬ЛщТФЦРРДФӯЧУNөДФУ»Ҝ·ҪКҪОӘsp3ФУ»ҜЈ»
Ј¬ёщҫЭVSEPRАнВЫЈ¬NH2ЈӯөДАнВЫ№№РНОӘХэЛДГжМеЈ¬Ттә¬БҪ¶Ф№В¶ФөзЧУЈ¬ЛщТФNH2ЈӯөДҝХјд№№РНОӘVРОЈ¬ТтјЫІгөзЧУ¶ФКэОӘ4Ј¬ЛщТФЦРРДФӯЧУNөДФУ»Ҝ·ҪКҪОӘsp3ФУ»ҜЈ»
(3)ёщҫЭөзёәРФКэЦөЈ¬H3NЈӯBH3ЦРөӘФӯЧУЙПөДЗвФӯЧУҙшХэөзәЙЈ¬ЕрФӯЧУЙПөДЗвФӯЧУҙшёәөзәЙЈ¬ЛщТФH3NЈӯBH3УлЛ®·ҙУҰКұЈ¬ЈӯBH3ЦРөДЗвФӯЧУУлЛ®·ўЙъ№йЦР·ҙУҰЙъіЙЗвЖшЈ¬ЈӯBH3ЧӘ»ҜОӘB(OH)4-ЈЁұдҝЙјтРҙОӘBO2-Ј©Ј¬ЛщТФ·ҙУҰөД»ҜС§·ҪіМКҪОӘЈәH3NЈӯBH3+2H2O = NH4BO2 +3H2Ј»
ЕрКЗөЪ5әЕФӘЛШЈ¬ФЪФӘЛШЦЬЖЪұнЦРО»УЪөЪ¶юЦЬЖЪөЪўуAЧеЎўpЗшЈ¬Жд»щМ¬ФӯЧУәЛНвөзЧУЕЕІјКҪОӘ1s22s22p1Ј¬јЫөзЧУЕЕІјКҪОӘ2s22p1Ј¬јЫөзЧУ№мөАұнҙпКҪОӘ![]() Ј»
Ј»
(4) ўЩёщҫЭAlH63ЈӯөД·ЦКэЧшұкҝЙТФЕР¶ПЖдФЪҫ§°ыЦРөДО»ЦГЈә8ёцФЪ¶ҘөгЎў4ёцФЪІаАвАвРДЎў4ёцФЪҫ§°ыМеДЪЈ¬УЙ·ЦМҜ·ЁҝЙөГТ»ёцҫ§°ыЦРә¬УР6ёцAlH63ЈӯЈ¬ЛщТФТ»ёцҫ§°ыЦРЛщә¬УРLi+өДёцКэОӘ18Ј»
ўЪУЙҪШГжөДұЯіӨ(![]() 801.7pmәН945.5pm)ҝЙЦӘХвКЗ№эҫ§°ыөЧГжіӨЦбөДәбҪШГж,МвДҝұнГчБЛХвёцҪШГжЦРТ»№Іә¬УР10ёцAlH63Јӯ Ј¬УЦ»ӯіц4ёцО»УЪ¶ҘөгЎў2ёцО»УЪАвРДөДAlH63ЈӯЈ¬ЛщТФРиТӘ»ӯіцөДКЗ4ёцО»УЪҫ§.°ыМеДЪөДAlH63ЈӯЈ¬ҪбәПAlH63ЈӯөД·ЦКэЧшұкЈ¬јҙҝЙҪ«ИұК§өДAlH63ЈӯІ№ідНкХыЈ¬өГөҪПВНјЈә
801.7pmәН945.5pm)ҝЙЦӘХвКЗ№эҫ§°ыөЧГжіӨЦбөДәбҪШГж,МвДҝұнГчБЛХвёцҪШГжЦРТ»№Іә¬УР10ёцAlH63Јӯ Ј¬УЦ»ӯіц4ёцО»УЪ¶ҘөгЎў2ёцО»УЪАвРДөДAlH63ЈӯЈ¬ЛщТФРиТӘ»ӯіцөДКЗ4ёцО»УЪҫ§.°ыМеДЪөДAlH63ЈӯЈ¬ҪбәПAlH63ЈӯөД·ЦКэЧшұкЈ¬јҙҝЙҪ«ИұК§өДAlH63ЈӯІ№ідНкХыЈ¬өГөҪПВНјЈә »т
»т
ўЫёщҫЭҫ§°ыГЬ¶И№«КҪЈә![]() Ј¬ҫ§°ыЦРM=54g/molЎўZ=6ЎўV=801.72
Ј¬ҫ§°ыЦРM=54g/molЎўZ=6ЎўV=801.72![]() 945.5
945.5![]() sin60
sin60![]() pm3Ј¬ЛщТФёГҫ§МеөДГЬ¶ИОӘ
pm3Ј¬ЛщТФёГҫ§МеөДГЬ¶ИОӘ![]() ЎЈ
ЎЈ


 МмМмПтЙПТ»ұҫәГҫнПөБРҙр°ё
МмМмПтЙПТ»ұҫәГҫнПөБРҙр°ё РЎС§Йъ10·ЦЦУУҰУГМвПөБРҙр°ё
РЎС§Йъ10·ЦЦУУҰУГМвПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝоСКЗТ»ЦЦРФДЬ·ЗіЈУЕФҪөДҪрКфЈ¬21КАјНҪ«КЗоСөДКАјНЎЈ
(1)TiO2ұЎДӨЦРІфФУёхДЬПФЦшМбёЯ№вҙЯ»Ҝ»оРФЎЈ»щМ¬TiФӯЧУөДјЫөзЧУЕЕІјНјОӘ___________ЎЈ
(2)ЛДТТҙјоСДЬФцјУПрҪәФЪҪрКфұнГжөДХіёҪРФЎЈЖдЦЖұёФӯАнИзПВЈәTiCl4+4CH3CH2OH+4NH3=Ti(OCH2CH3)4+4NH4ClЎЈ
ўЩTi(OCH2CH3)4ҝЙИЬУЪУР»ъИЬјБЈ¬іЈОВПВОӘөӯ»ЖЙ«НёГчТәМеЈ¬Ждҫ§МеАаРНОӘ_________ЎЈ
ўЪNәНOО»УЪН¬Т»ЦЬЖЪЈ¬OөДөЪ¶юөзАлДЬҙуУЪNөДөЪ¶юөзАлДЬөДФӯТтКЗ___________ЎЈ
ўЫNH4ClЦРҙжФЪөДЧчУГБҰУР________Ј¬NH4ClИЫ·РөгёЯУЪCH3CH2OHөДФӯТтКЗ________Ј¬Ti(OCH2CH3)4·ЦЧУЦРCФӯЧУөДФУ»ҜРОКҪҫщОӘ__________ЎЈ
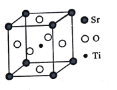
(3)оСЛбпИ(SrTiO3)ҝЙЧчөзЧУМХҙЙІДБПәНИЛФмұҰКҜЈ¬ЖдЦРТ»ЦЦҫ§°ыҪб№№ИзНјЛщКҫЎЈИфTiО»УЪ¶ҘөгО»ЦГЈ¬OО»УЪ__________О»ЦГЈ»ТСЦӘҫ§°ыІОКэОӘa nmЈ¬TiО»УЪOЛщРОіЙөДХэ°ЛГжМеөДМеРДЈ¬ФтёГ°ЛГжМеөДұЯіӨОӘ__________m(БРіцұнҙпКҪ)ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝҪрКфов(Mo)ФЪ№ӨТөәН№ъ·АҪЁЙиЦРУРЦШТӘөДЧчУГЎЈовөДіЈјы»ҜәПјЫОӘ+4Ўў+5Ўў+6ЎЈУЙовҫ«ҝу(ЦчТӘіЙ·ЦКЗMoS2)ЦЖұёөҘЦКовәНовЛбДЖҫ§Ме(![]() )Ј¬Іҝ·ЦБчіМИзПВНјЛщКҫЈә
)Ј¬Іҝ·ЦБчіМИзПВНјЛщКҫЈә
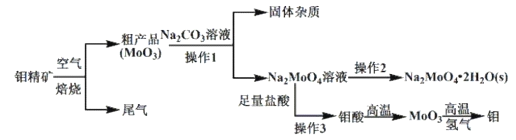
ТСЦӘЈәовЛбОўИЬУЪЛ®Ј¬ҝЙИЬУЪјоИЬТәЎЈ
»ШҙрПВБРОКМвЈә
(1)овҫ«ҝуФЪҝХЖшЦРұәЙХКұЈ¬·ўЙъөДЦчТӘ·ҪіМКҪОӘ_______________________ЎЈ
(2)овҫ«ҝуұәЙХКұЕЕ·ЕөДОІЖш¶Ф»·ҫі»бІъЙъОЈәҰЈ¬ЗлДгМбіцТ»ЦЦКөСйКТіэИҘёГОІЖшөД·Ҫ·Ё________________________ЎЈ
(3)ІЩЧч2өДГыіЖОӘ_____________________ Ўў№эВЛЎўПҙөУЎўёЙФпЎЈ
(4)КөСйКТУЙовЛбҫӯёЯОВЦЖMoO3Ј¬ЛщУГөҪөД№иЛбСОІДБПТЗЖчөДГыіЖКЗ_________ЎЈ
(5)ІЩЧч1ЦРЈ¬јУИлМјЛбДЖИЬТәід·Ц·ҙУҰәуЈ¬јоҪюТәЦРc(![]() )=0.80 molL1Ўўc(
)=0.80 molL1Ўўc(![]() )=0.05 molL1Ј¬ФЪҪбҫ§З°РијУИлBa(OH)2№ММеТФіэИҘИЬТәЦРөД
)=0.05 molL1Ј¬ФЪҪбҫ§З°РијУИлBa(OH)2№ММеТФіэИҘИЬТәЦРөД![]() ЎЈөұBaMoO4ҝӘКјіБөнКұЈ¬SO42өДИҘіэВККЗ_________ЎЈ[Ksp(BaSO4)=1.1
ЎЈөұBaMoO4ҝӘКјіБөнКұЈ¬SO42өДИҘіэВККЗ_________ЎЈ[Ksp(BaSO4)=1.1![]() 1010ЎўKsp(BaMoO4)=4.0
1010ЎўKsp(BaMoO4)=4.0![]() 108Ј¬ИЬТәМе»эұд»ҜҝЙәцВФІ»јЖЎЈ]
108Ј¬ИЬТәМе»эұд»ҜҝЙәцВФІ»јЖЎЈ]
(6)ұәЙХовҫ«ҝуЛщУГЧ°ЦГКЗ¶аІгұәЙХВҜЈ¬ПВНјОӘёчВҜІг№ММеОпБПөДОпЦКөДБҝөД°Щ·ЦКэ(![]() )ЎЈ
)ЎЈ
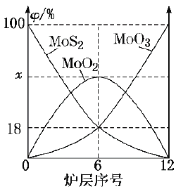
ўЩx=___________ЎЈ
ўЪұәЙХВҜЦРТІ»б·ўЙъMoS2УлMoO3·ҙУҰЙъіЙMoO2әНSO2Ј¬»№ФӯјБОӘ_________ЎЈИф·ҙУҰЦРЧӘТЖ3moleЈӯЈ¬ФтПыәДөД»№ФӯјБөДОпЦКөДБҝОӘ______________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДіҝЖСРИЛФұМбіцHCHOУлO2ФЪфЗ»щБЧ»ТКҜ(HAP)ұнГжҙЯ»ҜСх»ҜЙъіЙCO2ЎўH2OөДАъіМЈ¬ёГАъіМИзНјЛщКҫ(НјЦРЦ»»ӯіцБЛ HAPөДІҝ·ЦҪб№№Ј¬УГ18OұкјЗфЗ»щБЧ»ТКҜЦРөДфЗ»щСхФӯЧУ)ЎЈПВБРЛө·ЁХэИ·өДКЗ( )
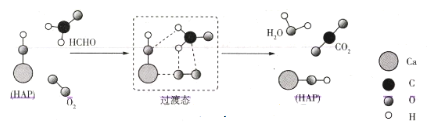
A.·ҙУҰОпөДјьДЬЦ®әНҙуУЪЙъіЙОпөДјьДЬЦ®әН
B.HAPёДұдБЛёГ·ҙУҰөДАъіМәНмКұдЈ¬јУҝмБЛ·ҙУҰЛЩВК
C.ҫӯ№эёГҙЯ»ҜСх»Ҝ№эіМәу18OИФИ»ФЪHAPЦР
D.·ҙУҰ№эіМЦРЈ¬МјФӯЧУУЙsp2ФУ»ҜұдОӘspФУ»Ҝ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝНј(1)КЗКөСйКТәПіЙдеұҪІўјмСйЖдІҝ·ЦЙъіЙОпөДЧ°ЦГЈ¬ПВБРЛө·ЁҙнОуөДКЗ( )

A.ұҪәНТәдеФЪAЦР·ўЙъөД·ҙУҰОӘИЎҙъ·ҙУҰ
B.КөСйЦРCЦРөДТәМеЦрҪҘұдОӘЗіәмЙ«Ј¬КЗТтОӘдеҫЯУР»У·ўРФ
C.DЎўEЎўFҫщҫЯУР·Аө№ОьөДЧчУГЈ¬ЖдЦРFІ»ҝЙТФУГНј(2)ЛщКҫЧ°ЦГҙъМж
D.DЦРКҜИпКФТәВэВэұдәмЈ¬EЦРІъЙъЗі»ЖЙ«іБөн
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ»ЖЙ«і¬Сх»ҜјШ(KO2)ҝЙЧчОӘУоЦж·Йҙ¬ІХөДСхФҙЎЈДіС§П°РЎЧйЙијЖТФПВКөСйМҪҫҝKO2өДРФЦКЈ¬Зл»ШҙрПа№ШОКМвЈә
ўс. МҪҫҝKO2УлЛ®өД·ҙУҰЈәИЎЙЩБҝKO2№ММеУЪКФ№ЬЦРЈ¬өОјУЙЩБҝЛ®ҝмЛЩІъЙъЖшЕЭЈ¬Ҫ«ҙш»рРЗөДДҫМхҝҝҪьКФ№ЬҝЪДҫМхёҙИјЈ»өОјУ·УМӘКФТәЈ¬ИЬТәПИұдәмәуНКЙ«ЎЈПтНКЙ«әуИЬТәЦРөОјУFeCl3ИЬТәЈ¬ІъЙъөДПЦПуОӘ____________________________________ЎЈ
ўт. МҪҫҝKO2УлSO2өД·ҙУҰЈә
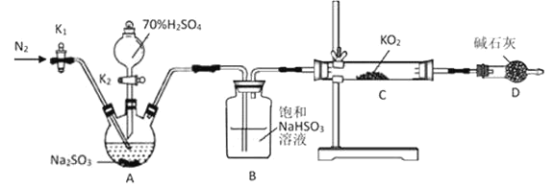
(1)ХэИ·өДІЩЧчТАҙОКЗ_________________________ЎЈ(ІЩЧчҝЙЦШёҙСЎФс)
ўЩҙтҝӘK1НЁИлN2Ј¬ІўО¬іЦТ»¶ОКұјдәу№ШұХ
ўЪКөСйНкіЙЈ¬ІрР¶Ч°ЦГ
ўЫјмІйЧ°ЦГЖшГЬРФЈ¬И»әуЧ°ИлТ©Ж·
ўЬҙтҝӘ·ЦТәВ©¶·»оИыK2
(2)AЧ°ЦГ·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪОӘ________________________ЎЈ
(3)УГЙПКцЧ°ЦГСйЦӨЎ°KO2УлSO2·ҙУҰЙъіЙO2Ўұ»№ҙжФЪІ»ЧгЈ¬ДгөДёДҪшҙлК©КЗ________ЎЈ
(4)ёДҪшәуФЩКөСйЈ¬ҙэKO2НкИ«·ҙУҰәуЈ¬Ҫ«Ч°ЦГCЦР№ММејУЛ®ИЬҪвЈ¬ЕдіЙ50.00mLИЬТәЈ¬өИ·ЦОӘMЎўNБҪ·ЭЎЈ
ўЩПтMИЬТәЦРјУИлЧгБҝөДСОЛбЛб»ҜөДBaCl2ИЬТәЈ¬ід·Ц·ҙУҰәуЈ¬өГіБөн4.66gЎЈ
ўЪҪ«NИЬТәТЖИлЧ¶РОЖҝЦРЈ¬УГ0.20mol ![]() L-1ЛбРФKMnO4ИЬТәөО¶ЁЈ¬өұіцПЦ___________ПЦПуКұЈ¬ҙпөҪөО¶ЁЦХөгЈ¬ҙЛКұПыәДЛбРФKMnO4ИЬТә20.00mLЎЈ
L-1ЛбРФKMnO4ИЬТәөО¶ЁЈ¬өұіцПЦ___________ПЦПуКұЈ¬ҙпөҪөО¶ЁЦХөгЈ¬ҙЛКұПыәДЛбРФKMnO4ИЬТә20.00mLЎЈ
ўЫТАҫЭЙПКцПЦПуәНКэҫЭЈ¬ЗлРҙіцЧ°ЦГCЦРЧЬ·ҙУҰөД»ҜС§·ҪіМКҪ___________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРОпЦКөДЦЖұёЈ¬І»·ыәП№ӨТөЙъІъКөјКөДКЗЈЁ Ј©
A.№ӨТөЙПУГөзҪвИЫИЪВИ»ҜГҫЦЖұёөҘЦКГҫ
B.№ӨТөЙПУГөзҪвұҘәНКіСОЛ®ЦЖұёВИЖш
C.№ӨТөЙПУГ¶юСх»Ҝ№иФЪёЯОВПВУлҪ№Мҝ·ҙУҰЦЖөГёЯҙҝ№и
D.№ӨТөЙПБ¶МъКұЈ¬іЈУГКҜ»ТКҜіэИҘМъҝуКҜЦРөД¶юСх»Ҝ№и
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ№ӨТөЙПТ»°гТФCOәНH2ОӘФӯБПәПіЙјЧҙјЈ¬ёГ·ҙУҰөДИИ»ҜС§·ҪіМКҪОӘЈәCO(g)+2H2(g)![]() CH3OH(g) ЎчH
CH3OH(g) ЎчH
ЈЁ1Ј©ёГ·ҙУҰөДҰӨH___0ЈЁМоЎ°ЈҫЎұЎўЎ°ЈјЎұ»тЎ°=ЎұЈ©ЎЈ
ЈЁ2Ј©ИфҪ«өИОпЦКөДБҝөДCOәНH2»мәПЖшМеідИләгОВәгИЭГЬұХИЭЖчЦРҪшРРЙПКц·ҙУҰЈ¬ПВБРКВКөДЬЛөГчҙЛ·ҙУҰТСҙпөҪЖҪәвЧҙМ¬өДКЗ___ЎЈ
A.ИЭЖчДЪЖшМеГЬ¶ИұЈіЦІ»ұд
B.»мәПЖшМеөДЖҪҫщПа¶Ф·ЦЧУЦКБҝІ»ұд
C.ЙъіЙCH3OHөДЛЩВКУлЙъіЙH2өДЛЩВКПаөИ
D.COөДМе»э·ЦКэұЈіЦІ»ұд
ЈЁ3Ј©өұ°ҙН¶БПұИ![]() =2Ј¬·ЦұрФЪP1ЎўP2ЎўP3С№ЗҝПВЈ¬ІвөГІ»Н¬ОВ¶ИПВЖҪәвКұCOөДЖҪәвЧӘ»ҜВКИзНј1ЎЈ
=2Ј¬·ЦұрФЪP1ЎўP2ЎўP3С№ЗҝПВЈ¬ІвөГІ»Н¬ОВ¶ИПВЖҪәвКұCOөДЖҪәвЧӘ»ҜВКИзНј1ЎЈ
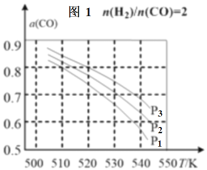
520KКұЈ¬Н¶БПұИ![]() =2(ЧЬОпЦКөДБҝОӘ3mol)Ј¬О¬іЦ·ҙУҰ№эіМЦРС№ЗҝP3І»ұдЈ¬ҙпөҪЖҪәвКұІвөГИЭЖчМе»эОӘ0.1LЈ¬ФтЖҪәвіЈКэK=___ЎЈ
=2(ЧЬОпЦКөДБҝОӘ3mol)Ј¬О¬іЦ·ҙУҰ№эіМЦРС№ЗҝP3І»ұдЈ¬ҙпөҪЖҪәвКұІвөГИЭЖчМе»эОӘ0.1LЈ¬ФтЖҪәвіЈКэK=___ЎЈ
ИфH2әНCOөДОпЦКөДБҝЦ®ұИОӘnЎГ1ЈЁО¬іЦ·ҙУҰ№эіМЦРС№ЗҝP3І»ұдЈ©Ј¬ПаУҰЖҪәвМеПөЦРCH3OHөДОпЦКөДБҝ·ЦКэОӘxЈ¬ЗлФЪНј2ЦР»жЦЖxЛжnұд»ҜөДКҫТвНј___ЎЈ
ЈЁ4Ј©јЧҙјәПіЙјЧГСөД·ҙУҰОӘЈә
ўс.2CH3OH(g)![]() CH3OCH3(g)+H2O(g)(Цч·ҙУҰ)
CH3OCH3(g)+H2O(g)(Цч·ҙУҰ)
ўт.2CH3OH(g)![]() C2H4(g)+2H2O(g)(ёұ·ҙУҰ)
C2H4(g)+2H2O(g)(ёұ·ҙУҰ)
·ҙУҰ№эіМЦРөДДЬБҝұд»ҜИзНј3ЛщКҫЎЈ
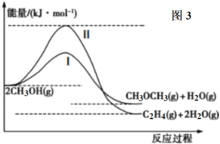
ўЩФЪТ»¶ЁОВ¶ИПВЈ¬ФЪәгИЭИЭЖчЦРҪшРРәПіЙјЧГСөД·ҙУҰЈ¬ІвөГCH3OCH3ә¬БҝЛжЧЕКұјдөДНЖТЖЈ¬ПИФцҙуәујхРЎЈ¬ЗлҪбәПЕцЧІАнВЫЛөГчФӯТтЈә___ЎЈ
ўЪФЪІ»ёДұдОВ¶ИөДЗ°МбПВЈ¬ЛөіцЖдЦРТ»ёцДЬФцҙуCH3OCH3СЎФсРФөДҙлК©Јә___ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВНјКЗijѧУКөСйКТҙУ»ҜС§КФјБЙМөкВт»ШөДЕЁБтЛбКФјБұкЗ©ЙПөДІҝ·ЦДЪИЭЎЈПЦУГёГЕЁБтЛбЕдЦЖ480 mL 1 molЎӨ LЈӯ1өДПЎБтЛбЎЈҝЙ№©СЎУГөДТЗЖчУРЈәўЩ ҪәН·өО№ЬўЪ ЙХЖҝўЫ ЙХұӯўЬ ІЈБ§°фўЭ Т©іЧўЮ БҝНІўЯ НРЕММмЖҪЎЈЗл»ШҙрПВБРОКМвЈә

ЈЁ1Ј©ФЪИЭБҝЖҝөДК№УГ·Ҫ·ЁЦРЈ¬ПВБРІЩЧчІ»ХэИ·өДКЗ________
AЈ®К№УГИЭБҝЖҝЗ°јмІйЛьКЗ·сВ©Л®
BЈ®ИЭБҝЖҝУГХфБуЛ®Пҙҫ»әуЈ¬ЕдЦЖИЬТәІ»РиТӘёЙФп
CЈ®ЕдЦЖИЬТәКұЈ¬Из№ыКФСщКЗ№ММеЈ¬°СіЖәГөДКФСщУГЦҪМхРЎРДө№ИлИЭБҝЖҝЦРЈ¬»әВэјУИлХфБуЛ®өҪҪУҪьұкПЯ1Ў«2cmҙҰЈ¬УГөО№ЬјУХфБуЛ®өҪұкПЯ
DЈ®¶ЁИЭәуёЗәГЖҝИыУГКіЦё¶ҘЧЎЖҝИыЈ¬УГБнТ»Ц»КЦНРЧЎЖҝөЧЈ¬°СИЭБҝЖҝөЯө№ТЎФИ¶аҙО
ЈЁ2Ј©ёГЕЁБтЛбөДОпЦКөДБҝЕЁ¶ИОӘ_________molЎӨ LЈӯ1ЎЈ
ЈЁ3Ј©ЕдЦЖПЎБтЛбКұЈ¬»№ИұЙЩөДТЗЖчУР________________ЎЈ
ЈЁ4Ј©ЕдЦЖ480mL 1molЎӨ LЈӯ1өДПЎБтЛбРиТӘУГБҝНІБҝИЎЙПКцЕЁБтЛбөДМе»эОӘ_______mLЎЈЛщРиБҝНІөД№жёсОӘ________ЎЈЈЁҝЙ№©СЎФсөДБҝНІУР5mLЎў10mLЎў20mLЎў50mLЎў100mLЈ©
ЈЁ5Ј©№эіМЦРөДІҝ·ЦІҪЦиЈ¬ЖдЦРУРҙнОуөДКЗ(МоРҙРтәЕ)____________ЎЈ

ЈЁ6Ј©¶ФЛщЕдЦЖөДПЎБтЛбҪшРРІв¶ЁЈ¬·ўПЦЖдЕЁ¶ИҙуУЪ1 molЎӨ LЈӯ1Ј¬ЕдЦЖ№эіМЦРПВБРёчПоІЩЧчҝЙДЬТэЖрёГЕЁ¶ИЖ«ёЯөДФӯТтУР___________ЎЈ
AЈ®¶ЁИЭКұЈ¬СцКУИЭБҝЖҝҝМ¶ИПЯҪшРР¶ЁИЭЎЈ
BЈ®Ҫ«ПЎКНәуөДБтЛбБўјҙЧӘИлИЭБҝЖҝәуЈ¬ҪфҪУЧЕҫНҪшРРТФәуөДКөСйІЩЧчЎЈ
CЈ®ИЭБҝЖҝУГХфБуЛ®ПҙөУәуОҙёЙФпЈ¬ә¬УРЙЩБҝХфБуЛ®ЎЈ
DЈ®ЧӘТЖИЬТәКұЈ¬І»ЙчУРЙЩБҝИЬТәИчөҪИЭБҝЖҝНвГжЎЈ
EЈ®¶ЁИЭәуЈ¬°СИЭБҝЖҝө№ЦГТЎФИәу·ўПЦТәГжөНУЪҝМ¶ИПЯЈ¬ұгІ№ідјёөОЛ®ЦБҝМ¶ИҙҰЎЈ
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com