
����Ŀ�����⻯��(NaAlH4)���л��ϳɵ���Ҫ��ԭ������ϳ���·����ͼ��ʾ��
![]()
��1�����⻯����ˮ�������ҷ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽΪ_____________________��
��2��AlCl3��NaH��Ӧʱ���轫AlCl3�����л��ܼ����ٽ��õ�����Һ�μӵ�NaH��ĩ�ϣ��˷�Ӧ��NaH��ת���ʽϵ͵�ԭ����__________________��
��3��ʵ����������ͼװ����ȡ��ˮAlCl3��
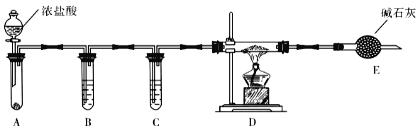
��A����ʢװ���Լ���_______________��
�ڵ�ȼD���ƾ���֮ǰ���ų�װ���еĿ������������______________________��
��4���ı�A��D�е��Լ��Ϳ����ø�װ����ȡNaH����װ���в������������Ƶõ�NaH�п��ܺ��е�����Ϊ____________
��5���������������װ�ã��ⶨ���⻯�ƴֲ�Ʒ(ֻ����NaH����)�Ĵ��ȡ�

�Ӽ�Լ�ԡ�ȷ�Կ��ǣ������˵�װ����_________(����)����ȡ15.6g��Ʒ��ˮ��ȫ��Ӧ��������ڱ�״���µ����Ϊ22.4L����Ʒ�����⻯�Ƶ���������Ϊ___________��(���������λ��Ч����)
���𰸡�NaAlH4+2H2O=NaAlO2+4H2�� ��Ӧ���ɵ�NaCl������NaH���棬��ֹ��AlCl3��NaH��һ����Ӧ����NaH�����ӻ�����������л��ܼ���ʹ��Ӧ�����ԽӴ���������Ӧ����������أ�������ء������ظ���ء�����������ơ��ȣ���Һ©������ʹA�з�����Ӧ����D�г�������ɫ����ʱ��ȼ�ƾ���Na2O2��0.69
��������
���⣨1�����⻯����ˮ�������ҷ�Ӧ������ԭ���غ���ж���������ƫ�����ƺ��������䷴Ӧ�Ļ�ѧ����ʽΪNaAlH4��2H2O=NaAlO2��4H2����
��2�����ڷ�Ӧ���ɵ�NaCl������NaH���棬��ֹAlCl3��NaH��Ӧ�Ľ��У����Է�Ӧ��NaH��ת���ʽϵ͡�
��3�������ڷ�Ӧ����Ҫ���ȣ���A�е��Լ�����ǿ�����ԣ����õ���KMnO4��KClO3��K2Cr2O7��Ca(ClO)2�ȡ�
�������������ɵ������ſ�����������Ǵ�Һ©������ʹA�з�����Ӧ����D�г�������ɫ����ʱ��ȼ�ƾ��ơ�
��4�����������Ʒ�Ӧ���ɹ������ƣ���NaH�п��ܺ��е�����ΪNa2O2��
��5�������������ʾ�����ˮ��Ӧ������������˿���ͨ������������������㴿�ȡ�����Ϊ��������ѹǿ��ȣ���Ҫ��ѹ©�������������˵�װ�����ң�����Ʒ�����⻯�Ƶ����ʵ�����xmol��NaH��ymol����54x��24y��15.6��4x��y��1�����x��0.2��������Ʒ�����⻯�Ƶ���������Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ǰ������Ԫ��A��B��C��D��E��Fԭ���������ε�������֪��A��B��D��C�ļ۵������������࣬A��B��Cͬ������C�Ǹ������е縺������Ԫ�أ�A�����������ӣ�E��δ�ɶԵ�������ǰ�����������ģ�����۵�������D��ͬ�� F������������Ϊ2���ڲ�ȫ�����������ö�Ӧ��Ԫ�ط��Żش���������⣺
��1��д��E�ļ۵����Ų�ʽ��______________��
��2����A�γɵĻ������У�A��ȡsp2�ӻ����ҷ�������С�Ļ�����Ϊ��д��ѧʽ��______________��
��3���������ʵ�����������йص���______________��
A. ��ȼ�����γ� B. A���⻯��ķе� C. B���⻯������ȶ���
��4��E3+������AB���γ������ӣ�����E3+��d2sp3��ʽ�ӻ����ӻ����ȫ��������AB���γ���λ������E3+����λ��Ϊ______________��1mol���������к���______________mol������
��5��F��D�γɵĻ����ᄃ����ͼ��F����λ��Ϊ______________�������ܶ�Ϊa g/cm3,NAΪ�����ӵ����������߳�Ϊ______________pm����1pm=10-10cm��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2CO3��Һ�����ᷴӦ�����е������仯ʾ��ͼ��ͼ�����з�Ӧ�������ȷ�Ӧ���ǣ� ��
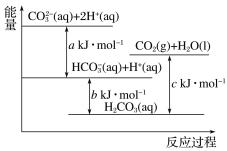
A. CO32��(aq)��H��(aq)=HCO3��(aq)
B. CO32��(aq)��2H��(aq)=CO2(g)��H2O(l)
C. HCO3��(aq)��H��(aq)=CO2(g)��H2O(l)
D. CO2(g)��H2O(l)=H2CO3(aq)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����Ȼ�ѧ����ʽ��
��H2(g)+1/2O2(g)��H2O(l) ��H =��285.8 kJ/mol
��H2(g)+1/2O2(g)��H2O(g) ��H =��241.8 kJ/mol
��C(s)+1/2O2(g)��CO(g) ��H =��110.5 kJ/mol
��C(s)+O2(g)��CO2(g) ��H =��393.5 kJ/mol
�ش��������⣺
��1��H2��ȼ������H��_______��C��ȼ����Ϊ__________��
��2��ȼ��1 g H2����Һ̬ˮ���ų�������Ϊ_________��
��3����֪CO��ȼ����Ϊ283.0 kJ/mol������CO��H2��CO2��ɵĻ������116.8 L(��״��)����ȫȼ�պ�ų�������Ϊ867.9 kJ��������18 gҺ̬ˮ������������H2Ϊ_________L��CO�ڻ�������е��������ԼΪ_________(��ȷ��С�����һλ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ϡ����ķ�Ӧ�У���֪10sĩ�����Ũ�ȼ�����0.6 mol/L�������Ƿ�Ӧ��������Һ����ı仯����10s��������������ƽ����Ӧ������( )
A. 0.02 mol/(Lmin)B. 1.2 mol/(Lmin)
C. 1.8mol/(Lmin)D. 0.18 mol/(Lmin)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���������ȷ���ǣ� ��
A.��ˮ�������Ȼ�̼�;ƾ��Ļ����
B.�������ʱ������ֹͣ���ȣ��ر�����ˮ
C.����һ�����ʵ���Ũ�ȵ�������Һʱ������Ͳ��ȡһ�������Ũ���ᵹ���ձ��������� ��ˮϴ����Ͳ2��3�Σ�����ϴ��Һһ�������ձ���ϡ��
D.�����ڳ���ֽ�ϳ�ȡ��NaOH�������ձ����ܽ⣬����ȴ����ת��������ƿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ڸ����������ܷ����������ж���ȷ����������Ӧ�����ӷ���ʽҲ��ȷ����
ѡ�� | ���� | ������ | ���ӹ����жϼ���Ӧ���ӷ���ʽ |
A | �μӰ�ˮ | Na+��Al3+��Cl-��NO3- | ���ܹ��棬Al3++3OH-=Al(OH)3�� |
B | pH =1����Һ | Fe2+��Al3+��SO42-��MnO4- | ���ܹ��棬5Fe2++MnO4-+8H+= Mn2++5Fe3++4H2O |
C | ��ˮ�������H+Ũ��Ϊ1��10-12 mol��L-1 | NH4+��Na+��NO3-��Cl- | һ���ܹ��棬NH4++ H2O |
D | ͨ������SO2���� | K+��Na+��ClO-��SO42- | ���ܹ��棬2C1O- +SO2+H2O=2HC1O+SO32- |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ����������������A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2��1��1��1�ֱ��γ����ֻ�������ҡ�D��A��ԭ�Ӹ�����3��2�γɻ��������E�ǵؿ��к������������Ԫ�ء�����������Ϣ�ش��������⣺
(1)BԪ�������ڱ��е�λ����_________________��
(2)A���ʵĵ���ʽ_________________��
(3)D�ļ������ӵ����ӽṹʾ��ͼ_________________��
(4)A��B��C��D��E����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳����(��Ԫ�ط�����д)_________��
(5)�õ���ʽ��ʾC2B2���γɹ���___________________________________��
(6)д��E�ĵ�����CԪ�ص�����������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д��CH4��Cl2�ڹ��յ�����������CH3Cl�Ļ�ѧ��Ӧ����ʽ_________
��2��д����ͼ��ʾԭ��صĵ缫����ʽ��
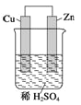
������_______________________
������_______________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com