
����Ŀ��ǰ������Ԫ��A��B��C��D��E��Fԭ���������ε�������֪��A��B��D��C�ļ۵������������࣬A��B��Cͬ������C�Ǹ������е縺������Ԫ�أ�A�����������ӣ�E��δ�ɶԵ�������ǰ�����������ģ�����۵�������D��ͬ�� F������������Ϊ2���ڲ�ȫ�����������ö�Ӧ��Ԫ�ط��Żش���������⣺
��1��д��E�ļ۵����Ų�ʽ��______________��
��2����A�γɵĻ������У�A��ȡsp2�ӻ����ҷ�������С�Ļ�����Ϊ��д��ѧʽ��______________��
��3���������ʵ�����������йص���______________��
A. ��ȼ�����γ� B. A���⻯��ķе� C. B���⻯������ȶ���
��4��E3+������AB���γ������ӣ�����E3+��d2sp3��ʽ�ӻ����ӻ����ȫ��������AB���γ���λ������E3+����λ��Ϊ______________��1mol���������к���______________mol������
��5��F��D�γɵĻ����ᄃ����ͼ��F����λ��Ϊ______________�������ܶ�Ϊa g/cm3,NAΪ�����ӵ����������߳�Ϊ______________pm����1pm=10-10cm��

���𰸡�3d54s1 C2H4 A 6 12 4 ![]()
��������
���Ȿ�⿼�����ʵĽṹ�����ʡ��漰Ԫ�ص��ƶϣ��۵����Ų�ʽ����д���������λ����ȷ���ͦҼ��ļ��㣬�����ķ����ͼ��㡣E��δ�ɶԵ�������ǰ�����������ģ�EΪCrԪ����F��ԭ����������E��F������������Ϊ2���ڲ�ȫ��������F��̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s2��FΪZnԪ����E�ļ۵�����Ϊ6��D�ļ۵�������E��ͬ��D�ļ۵�����Ϊ6��A��B��Cͬ���ڣ�A��B��C��Dԭ���������ε�����A��B��D��C�ļ۵���������������A��B��CΪ�ڶ����ڣ�DΪ�������ڣ�DΪSԪ�أ�A��B��CΪ�ڶ�������C�Ǹ������е縺������Ԫ����CΪFԪ�أ�A�ļ۵�����С��B��B�ļ۵�����С��6��A�����������ӣ���AΪCԪ�أ�BΪNԪ�ء�
��1��EΪCr��Cr��ԭ������Ϊ24������ԭ�Ӻ�������Ų����ɣ�E�ĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1���۵����Ų�ʽΪ3d54s1��
��2��AΪCԪ�أ���A�γɵĻ�������A��ȡsp2�ӻ�����������С�Ļ�����ΪCH2=CH2����ѧʽΪC2H4��
��3��A����ȼ��������Ȼ����ˮ�ڸ��µ�ѹ�������γɵ����״�����ʣ���ȼ����ˮ���Ӽ�ͨ������γ���״�ṹ����������CH4���ӻ�H2O���ӣ���ȼ�����γ�������йأ�B��AΪCԪ�أ�C�ĵ縺�Խ�С��A���⻯����Ӽ䲻�γ��������е�ĸߵ�������أ��뷶�»����йأ�C��BΪNԪ�أ�B���⻯������ȶ����빲�ۼ��ļ����йأ�����Ӽ�����أ�������йص���A����ѡA��
��4��EΪCr��Cr3+��d2sp3��ʽ�ӻ����γ�6���ӻ�������ӻ����ȫ��������AB-��CN-���γ���λ����Cr3+����λ��Ϊ6��6����λ��ȫΪ�Ҽ�������CN-�ĵ���ʽΪ![]() ��1������CN-��1���Ҽ���2���м�����1mol��������[Cr��CN��6]3-�к��еĦҼ�Ϊ��6+6
��1������CN-��1���Ҽ���2���м�����1mol��������[Cr��CN��6]3-�к��еĦҼ�Ϊ��6+6![]() 1��mol=12mol��
1��mol=12mol��
��5��FΪZn��DΪS��������̯������������С����ĸ���Ϊ4������ĸ���Ϊ8![]() +6
+6![]() =4���þ���Ļ�ѧʽΪZnS��Zn��S����λ����ȣ��ɾ�������С�������λ��Ϊ4����F����λ����Ϊ4��1mol���������Ϊ��65+32��g=97g��1mol��������Ϊ
=4���þ���Ļ�ѧʽΪZnS��Zn��S����λ����ȣ��ɾ�������С�������λ��Ϊ4����F����λ����Ϊ4��1mol���������Ϊ��65+32��g=97g��1mol��������Ϊ![]() =
=![]() cm3�����������Ϊ4
cm3�����������Ϊ4![]() ��
��![]() cm3
cm3![]() NA��=
NA��=![]() cm3�������ı߳�Ϊ
cm3�������ı߳�Ϊ![]() cm=
cm=![]() 1010pm��
1010pm��


 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������Ԫ��,���������������������������Ҫ����,ʵ��С����еⵥ�ʵ��Ʊ���
���������ϣ�����������,I2�ᷢ���绯��Ӧ����I-��IO3-,����������, I-��IO3-�ֻᷢ�����з�Ӧ����I2������ˮ�е��ܽ��Ϊ0.029g��
�������ȡ���Ժ���Ϊԭ��,�������²������ʵ�顣

��1������B�����ķ�Ӧ����������,��һ�����пɹ�ѡ�õ��Լ�: ��Cl2����Br2����ϡ�����H2O2,������Ⱦ�Ƕȿ��ǣ�����Ϊ�����Լ���______�����ţ�,��Ӧ��I-ת��ΪI2 �����ӷ�Ӧ����ʽΪ________��
��2������C��ʹ�õ��������õ�������_______,ʹ�ø�����ʱ,��һ��������_______________��
��3����ͬѧ�����Ϻ���CCl4�ж�,�������Ҵ�����,���жϸ������Ƿ����,ԭ����_______________��
��4������ķ��룩�õ���I2��CCl4��Һ��,������ͼװ�ý��е����ȡ�������ܼ���

ͼ�����������Դ���,�ֱ�����_________����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����![]() ����
����![]()
A. ![]() ��
��![]() ��
��![]() �У�����ԭ�Ӷ����������8���ӵ��ȶ��ṹ
�У�����ԭ�Ӷ����������8���ӵ��ȶ��ṹ
B. ��Ԫ�����ڱ��н����ͷǽ������紦�����ҵ��뵼�����
C. �ɷǽ���Ԫ����ɵĻ�����һ���ǹ��ۻ�����
D. �ڢ�A��Ԫ�غ͵ڢ�A��Ԫ�ص�ԭ��֮�䶼���γ����Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У�ˮ������ղ����в����������ǵ���( )
A.����B.����C.��ά��D.��֬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��698Kʱ����VL���ܱ������г���2molH2(g)��2molI2(g)��������Ӧ��H2(g)��I2(g)![]() 2HI(g)����H����26.5kJ��mol��1����ø����ʵ����ʵ���Ũ����ʱ��仯�Ĺ�ϵ��ͼ��ʾ��
2HI(g)����H����26.5kJ��mol��1����ø����ʵ����ʵ���Ũ����ʱ��仯�Ĺ�ϵ��ͼ��ʾ��
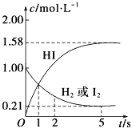
��ش��������⣺
(1)V��______��
(2)�÷�Ӧ�ﵽ����ȵ�ʱ����_______����ʱ����ƽ����Ӧ����v(HI)��________��
(3)����˵���п���˵����Ӧ2HI(g)![]() H2(g)��I2(g)�Ѵﵽƽ��״̬����_____��
H2(g)��I2(g)�Ѵﵽƽ��״̬����_____��
A.��λʱ��������nmolH2��ͬʱ����2nmolHI
B.�¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯
C.����һ������������ƽ����Է����������ٱ仯
D.�¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯
(4)�÷�Ӧ�ﵽƽ��״̬ʱ��__________(����ա��ų���)������Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Է���������̼������ʵ����ɫ�������������ã����ڼ�������Ҳ������Ҫ���塣
��1������β������Ҫ��Ⱦ��ΪNO����H2����ԭNO���Դﵽ������Ⱦ��Ŀ�ġ�
��֪��2NO(g) ![]() N2(g)��O2(g) ��H=��180.5 kJ��mol��1
N2(g)��O2(g) ��H=��180.5 kJ��mol��1
2H2O(l)===2H2(g)��O2(g) ��H=��571.6 kJ��mol��1
д��H2(g)��NO(g)��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽ��______________��
��2��ij�о�С��ģ���о����£���2 L�����ܱ������г���2 mol NO������Ӧ2NO(g) ![]() N2(g)��O2(g)���ڲ�ͬ���¶��£���Ӧ���������ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ��
N2(g)��O2(g)���ڲ�ͬ���¶��£���Ӧ���������ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ��

��T2�£���0��5 min�ڣ�v(O2)=______________mol��L��1��min��1�����¶��·�ӦN2(g)��O2(g) ![]() 2NO(g)��ƽ�ⳣ��K=______________��
2NO(g)��ƽ�ⳣ��K=______________��
���÷�Ӧ���е�M��ų�������______________���е�W��ų�������(��������������������=��)��
M��ʱ�ټ���һ����NO��ƽ���NO��ת����______________(���������������С������������)��
����Ӧ��ʼ���ﵽƽ��Ĺ����У����������и�����仯����______________(�����)��
a�����������ܶ� b���淴Ӧ����
c����λʱ���ڣ�N2��NO��������֮�� d�������ƽ����Է�������
��3��������Ϊһ������ȼ�ϣ�����������������䡣����������Ҫѡ����ʵĴ�����ϣ������Ͻ���һ�������¿����������γ��⻯�LaNi5(s)��3H2(g) ![]() LaNi5H6(s) ��H��0����ʹLaNi5H6(s)�ͷų���̬�⣬����ƽ���ƶ�ԭ�����ɸı��������______________(����ĸ���)��
LaNi5H6(s) ��H��0����ʹLaNi5H6(s)�ͷų���̬�⣬����ƽ���ƶ�ԭ�����ɸı��������______________(����ĸ���)��
A������LaNi5H6(s)���� B�������¶�
C��ʹ�ô��� D����Сѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�ΪԪ�����ڱ���һ���֣�����Ԫ��![]() �ڱ��е�λ�ã���Ҫ��ش��������⣺
�ڱ��е�λ�ã���Ҫ��ش��������⣺
�������� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
һ |
| |||||||
�� |
|
|
| |||||
�� |
|
|
|
|
|
��1����Ԫ��![]() ������õĽ���Ԫ����______
������õĽ���Ԫ����______![]() ��Ԫ������
��Ԫ������![]() ������õķǽ���Ԫ��������______
������õķǽ���Ԫ��������______![]() ��Ԫ������
��Ԫ������![]()
��2���õ���ʽ��ʾԪ��![]() ��
��![]() �γɵ�ԭ�Ӹ�����Ϊ1��2�Ļ����� _______________��Ԫ�����������γɵĻ�ѧ����������___________________��
�γɵ�ԭ�Ӹ�����Ϊ1��2�Ļ����� _______________��Ԫ�����������γɵĻ�ѧ����������___________________��
��3��![]() ��
��![]() ����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳����______
����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳����______![]() ��Ԫ�ط��ű�ʾ
��Ԫ�ط��ű�ʾ![]() ��
��
��4��Ԫ��![]() ��
��![]() �����γɵ���̬�⻯����ȶ���___________
�����γɵ���̬�⻯����ȶ���___________![]() ��ѧʽ��ʾ
��ѧʽ��ʾ![]() ��Ԫ��
��Ԫ��![]() ��
��![]() ��
��![]() ������������Ӧ��ˮ�����������ǿ������˳�� ___
������������Ӧ��ˮ�����������ǿ������˳�� ___![]() ��ѧʽ��ʾ
��ѧʽ��ʾ![]()
��5��д��Ԫ��![]() ��
��![]() ������������ˮ�������Ӧ�����ӷ���ʽ��______________��
������������ˮ�������Ӧ�����ӷ���ʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һС����Ͷ�뵽������Һ�У������������壬����������ɫ��������(����)
A.�Ȼ�þ��ҺB.����������ҺC.����ͭ��ҺD.�Ȼ�����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����⻯��(NaAlH4)���л��ϳɵ���Ҫ��ԭ������ϳ���·����ͼ��ʾ��
![]()
��1�����⻯����ˮ�������ҷ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽΪ_____________________��
��2��AlCl3��NaH��Ӧʱ���轫AlCl3�����л��ܼ����ٽ��õ�����Һ�μӵ�NaH��ĩ�ϣ��˷�Ӧ��NaH��ת���ʽϵ͵�ԭ����__________________��
��3��ʵ����������ͼװ����ȡ��ˮAlCl3��
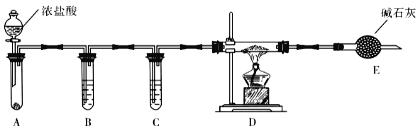
��A����ʢװ���Լ���_______________��
�ڵ�ȼD���ƾ���֮ǰ���ų�װ���еĿ������������______________________��
��4���ı�A��D�е��Լ��Ϳ����ø�װ����ȡNaH����װ���в������������Ƶõ�NaH�п��ܺ��е�����Ϊ____________
��5���������������װ�ã��ⶨ���⻯�ƴֲ�Ʒ(ֻ����NaH����)�Ĵ��ȡ�

�Ӽ�Լ�ԡ�ȷ�Կ��ǣ������˵�װ����_________(����)����ȡ15.6g��Ʒ��ˮ��ȫ��Ӧ��������ڱ�״���µ����Ϊ22.4L����Ʒ�����⻯�Ƶ���������Ϊ___________��(���������λ��Ч����)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com